Introduction
This experiment involves studying the heat required to melt ice when energy is transferred to it. The analysis is performed in a calorimeter, an insulated apparatus that allows the amount of heat transferred according to the second law of thermodynamics, from a hotter body to a colder body, to be studied (Lotha, 2023). In addition, the experiment utilizes the principle of energy conservation, which states that all the heat lost by the calorimeter setup will be transferred directly to the ice, causing it to transition into its liquid state. Data Table 1 shows the data obtained from direct measurements. Table 2 contains information about the change in water temperature every 30 seconds.
Table 1: Results of direct measurements during the experiment
Table 2: Results of temperature measurements at equal time intervals
Results
Calculations
To calculate the heat required to achieve ice melting, we must assume that the calorimeter was completely sealed, meaning that no heat escaped or entered the experimental setup. Mathematically, this is reflected in the equation [1].
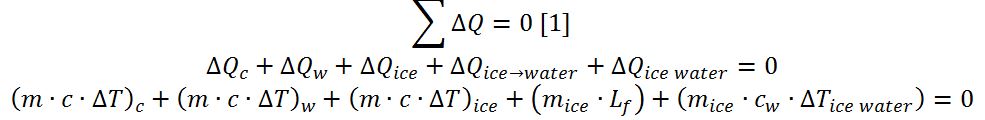
This equation can be solved with respect to the heat of fusion of water, as shown in [2]:
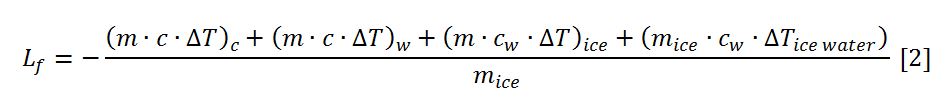
All data are known, so they can be substituted in [2] to find the heat of fusion of water, as shown in [3]:
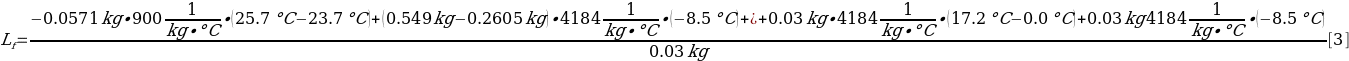
Before ice was added to the calorimeter cup, there was 0.2885 kg of water (0.549 kg-0.2605 kg). During the experiment, 0.03 kg of ice was used. Additional parts of the calorimeter are not considered because their masses can be considered negligibly small compared to the mass of water or ice. The resulting heat of the fusion of water generally meets expectations and is only 9.25% lower than the reference value (LibreTexts, 2020). Thus, the calculation can be considered successful.
Graph
Figure 1 illustrates the graphical relationship between water temperature and time. As can be seen, the temperature initially drops sharply: this drop is caused by the transfer of heat from the water to the ice, resulting in a local drop in temperature for a short time. Subsequently, the temperature reached a plateau, which is associated with the ice transition to the liquid state (its melting), but immediately afterward, the temperature began to rise.
Further temperature growth is related to the setting of the broken balance between ice and water, which tends to approach room temperature with the flow. Based on these states, the graph takes the form of falling, unchanging, and finally a continuously rising line. This shape can confirm the assumption that there was no heat exchange between the environment and the calorimetric system, i.e., the tightness of the experimental setup is confirmed.
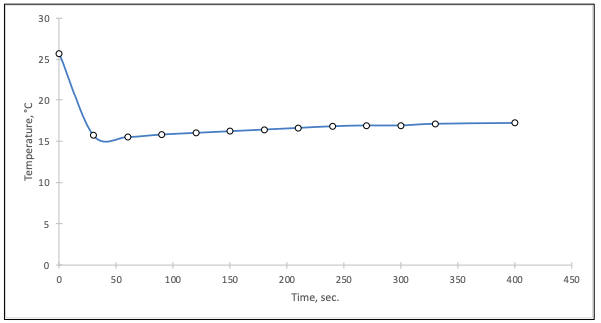
According to Figure 1, the initial temperature, Ti, was equal to 25.7, but the endpoint, Tf, was fixed at 17.2. Hence, the temperature difference between the initial and final state was -8.5 °C.
Conclusion
In the laboratory work, a calorimeter was used to determine the heat of water fusion. The ice of known mass was added to water, initially resulting in a sharp decrease in temperature due to the second law of thermodynamics, followed by a plateau transition, indicating the ice melting process. When all the ice was melted, the temperature rose until it reached equilibrium with the surrounding temperature. The obtained value of the heat of fusion of water was only 9.25% different from the reference value, so the laboratory work performed can be considered successful.
Reference
LibreTexts. (2020). Energy and phase transitions. LibreTexts Chemistry. Web.
Lotha, G. (2023). Second law of thermodynamics. Britannica. Web.