Abstract
The primary aim of this project was to carry out a comparative analysis of the three serological tests; Solid Phase, Gel Card, and Tube agglutination concerning their use in the detection of antibodies. The gel card and tube agglutination were used in this analysis for comparative purposes. As such, the project focused on the use of monolayer technology for the detection of various antibodies with a lot of consideration on the specificity, sensitivity, and endpoint stability of assays.
For many years, agglutination tests have been used although they have numerous drawbacks such as standardization and issues of control. As such, the introduction of the gel card as well as the solid phase technology is considered an improvement in the process of detecting antibodies due to the techniques’ high specificity and sensitivity as well as stable endpoints. Therefore, the experiment was carried out to offer comparative analysis to establish the most efficient method for blood banks.
Several phenomenal reagent red blood cells and well-known antibodies (anti-Fya, anti-E, anti-K, and anti-JKa) were used in this experiment for the SPRCA, Gel Card, and Tube agglutination methods. The results from all the tests were compared.
It was evident that the gel card and Solid Phase methods have high specificity, sensitivity as well as stable endpoints. These two methods are preferable for batch testing and screening.
Introduction
The advancement in technology has greatly improved the field of medicine as far as diagnosis and treatment is concerned. For example, the technological revolution that has been introduced globally, and the emergence of disruptive technology have slowly changed the landscape of the red-cell testing framework. According to Knight and de Silva, the application of the tube technique, which became a possibility in the mid-40s, has been known as a breakthrough that heralded a new era in managing the process of screening for the production of red-cell antibodies. As a rule, the first tests implied the use of the saline medium; at present, however, the application of the techniques based on the use of albumin and proteolytic enzymes as the means of testing for compatibility has become common practice. One must also give credit to the recent shift toward drawing a line between the tools that permit selecting the antibodies that can be deemed as clinically significant and those that do not.
As a result, the ones that do not have any potential effect on the outcomes of the analysis or the identification of changes in the patient’s health status can be dismissed in the course of the analysis. The given option helps avoid cluttering the analysis with extra information and thus contributes to a faster and a more efficient study. Currently, the Indirect Anti-globulin Test (IAT) is used as the primary tool for carrying out the corresponding analysis and locating the presence of antibodies in the red blood cells. When addressing the positive aspects of the testing system, one must mention the fact that IAT provides premises for a much more accurate evaluation and, therefore, a significant increase in the sensitivity of the tool.
A more recent study points to the fact that a significant leap has been made in the process of identifying the presence of antibodies in red blood cells. Apart from the solid-phase testing tool described above, the application of the methods, such as a parallel, blinded comparison of tube PEG, as well as the usage of automated methods, has been introduced into the framework. As the comparison made by Bunker, Thomas, and Geyer has shown quite explicitly, the PEG screening can be deemed as the most cost-efficient tool.
While the findings of the study can be deemed somewhat surprising given the previously mentioned properties of the solid-phase testing tool, one must admit that the PEG screening provides the basis for the most sensible use of the available resources while at the same time allowing one to retrieve quite accurate results. It should be noted, though, that the manual SPRCA test delivered results of higher precision compared to PE. The identified outcome was quite expected because the SPRCA tool as a variation of the manual test helped retrieve a significantly larger number of the false-positive screen test results, as the researchers explain.
The solid-phase approach is often mentioned alongside the strategy known as the tube-low-ionic-strength solution additive indirect antiglobulin test. It should be borne in mind, however, that the recent study on the subject matter considered only the detection of alloantibodies; in other words, the analysis implied the identification of solely the diseases that can be contracted from other people. At this point, one must bring up the fact that the current information regarding the sensitivity of microtube systems, which can be found in contemporary studies, is rather conflicting and, therefore, cannot provide a proper background for a complete analysis of the tool, as Weisbach et al. explain. According to the research outcomes, the Capture-R Ready-Screen tool allowed for retrieving the results of the highest sensitivity rate, which meant that it is significantly more effective than the manual reading framework. The latter showed a rather high rate of data omission in the process, therefore reducing the accuracy levels and causing the research process to become convoluted: “By automated reading of Solid screen II results, four weak-positive reactions detectable by manual reading were missed (two anti-Jka, one anti-D, and one anti-CD)’’.
This paper provides a comparative analysis of a lab report on the detection of antibodies through various methods with a lot of focuses on assay development and validation of SPRCA compared to other techniques.
Methodology and Materials
Materials
Use of Solid Phase Red Cell Adherence SPRCA to detect antibodies
The system of Solid Phase serological testing comprises of microtiter plates whose walls are usually coated with stroma extracted from the reagent cells (RBCs). Often, the reagent cells used ought to be of a phenotype that is known. Usually, the testing panels are readily available from manufacturers and in the process of testing; patient plasma, as well as Low Ionic Strength Solution (LISS), are added to the panels. An indicator, often RBCs with IgG coating is added to demonstrate the availability of antibodies under investigation. However, the surplus plasma should be washed off well from each microtiter before the addition of the indicator. All the materials that are needed for Solid Phase Red Cell Adherence assay are shown in the table below.
Table 2: Showing materials that are used for SPRCA.
Use of Tube and BioVue methods to detect antibodies
The BioVue and Serological Tube methods are types of agglutination assays which make use of testing tubes, waster baths and centrifuges in the detection of antibodies. BioVue centrifuges as well as BioVue poly-specific cassettes (cards) are used in for the BioVue method. The table below provides a comprehensive summary of the materials that are required for the BioVue test method.
Table 3: Showing materials that are used for BioVue and tube agglutination tests for the detection of antibodies.
Equipment
This comparative experiment involves serological tests that use common equipment that is found in the serology laboratory. The table below summarizes the equipment needed for this experiment.
Table 4: Showing various equipment needed for the equipment.
Reconstituted Buffers and Reagents
Several buffers and reagents were used for this experiment. The table below provides a comprehensive summary of the buffers and reagents that were used for this experiment.
Table 5; Showing the name and constituents of buffers and reagents used in this experiment.
Procedures of preparation and dilutions of any buffers and reagents
All the buffers and reagents used were prepared as well as diluted based on their individual use during the experiment. Highlighted below are the steps used in the preparation and dilutions of all the reagents used in this experiment.
Table 6: Preparation and dilution of buffers and reagents.
Methodologies
Several methods are used in for the detection of antibodies, which include the ABO Grouping-Solid Phase Assay method, Gel Card (BioVue) method, Tube Method, as well as the Solid Phase method. This section provides an overview of the procedures adopted for each method used in this experiment.
Solid Phase Method
Formation of Monolayer in Solid Phase Red Cell Assay
A monolayer of RBCs is necessary in the solid phase assay, and it can be formed whenever the plasma under investigation comprises of antibodies which have a capability of binding with the antigens affixed to the walls of the microtiter. In a case a monolayer forms, it is a likely indication of the presence of the antibodies under investigation. However, the failure of the monolayer to form symbolizes that the test is negative; an implication that the necessary antibodies are not present. As such, a delineated button is formed at the microplate wall’s center.Therefore, it is important to carry out various procedures to determine the presence of any suspected antibodies which is indicated by the absence or presence of a monolayer. As such, the test for the formation of monolayer involves the use of 0.2 per cent of red cell suspension. The suspension is prepared through the addition of about 40µl of 3 per cent RNC stock suspension to about 560µl of CFB. Alternatively, the suspension can be prepared through the addition of 150 µl of 0.8 per cent stock solution of RBC to 450 µl of CFB.
The addition of 50 µl of 0.2 per cent of the cell suspension into standard polystyrene microplate wells in the solid phase assay led to the formation of monolayers. The plate used was then centrifuged 350 RCF for about 3 minutes, after which the appearance of the monolayer was checked. In other ways, the microplates can be stored in a wet chamber that contains CFB to be used later after the centrifuging process.
Solid phase monolayer test
To test for the monolayer formation in the solid phase assay, microtiter wells, the test plasma, and Low Ionic Strength Solution are required. The solid phase red cell assay starts through the addition of Low Ionic Strength Solution (LISS) to the microtiter well. Also, the plasma under investigation is added to the microtiter well. In the case of the current experiment, 100µl of Low Ionic Strength Solution was mixed with 100µl of test serum and added to the microplate well. Samples of serum were used for the test on the presence of antibodies such as anti-E, anti-Jka, anti-K, as well as anti-Fya. After all the reagents and buffers were added to the microplate well, it was incubated under a temperature of 37° C for about 20 minutes. Non Buffered Saline was used to wash the microplate well gently for a couple of times before adding 50µl of 0.2 per cent AHG CC and 50µl of ½ strength AHG to each microplate. After all the reagents were added, a plastic cover was used to prevent the contents of the well from spilling off in the process of centrifuging the microplate at 500 RCF for about 2 minutes. After centrifugation, the results were read to establish whether or not a certain antibody was present.
Microplate longevity test
Empirical studies have shown that the results obtained from the Solid Phase Red Cell Assay are reliable since they are highly stable. Such a characteristic is important in that the results can be kept for reference at a later date. To test the stability of the monolayer formed through the Solid Phase assay method, the monolayers obtained were kept for four weeks under a temperature of 4° C. To ascertain the level of stability of the monolayers, it was important to check the layers after a week through centrifugation. The loss of stability of the monolayers can be determined by assessing whether or not there is any sediment found at the bottom of the plate upon centrifugation. Positive results indicate loss of stability, while negative results show that the monolayers are stable.
Using Papain to test the sensitivity of the Solid Phase method
The titration method can be used to test the sensitivity of the solid Phase Red Cell Assay method. The following procedure is used:
Materials and reagents
- Tested anti-Fya antibodies.
- Test tubes in a rack.
- 500µl of NBS.
- Pipettes.
- Pipette tips.
- Serum sample.
- 0.2 per cent cell diluent.
- 100µl of Low Ionic Strength Solution.
- Microplate wells.
- ½ strength AHG.
- 50µl AHG CC.
Procedure
- Use samples of serums that have been tested for anti-Fya antibodies through the use of the SRPCA method.
- Using a pipette, draw about 500µl and put it in the test tube.
- Use a different pipette tip to draw 500µl of a serum sample to the test tube and mix thoroughly.
- 150µl of 0.8 per cent red cell stock suspension added to 450µl of NBS buffer was used to make a 0.2 per cent the cell diluent.
- Different wells were used to hold 100µl of sera and 100µl of Low Ionic Strength Saline.
- The wells were incubated at a temperature of 37° C for 25 minutes before being washed using NBS for about 5 times.
- After the wells are washed, 50µl of AHG CC and 50µl ½ strength AHG was added to the plates and centrifuged at 500 RpM for 2 minutes before reading the results.
A specificity test was carried out on the Solid Phase method through the use of anti-Fya with anti-E serum, two positive cell monolayer and two negative monolayer cell samples. In this specificity method, highly concentrated titrations were found to be positive. However, lowly concentrated titers were negative and thus, there was no formation of monolayers.
Detection of Antibodies using the Tube method
In this serological test, reaction tubes are used to contain the reagents and testing samples. In this experiment, tubes were first labeled according to the panel cells. A drop of 3% panel cell suspension and two drops of the patient’s test serum were added to each of the tubes.
A low speed (500RPM) was used to centrifuge the tubes in the IS phase for between 15 and 20 seconds before checking them for hem-agglutination.
The LISS phase was incubated at 37ºC to enable the occurrence of hem-agglutination to occur. Next, 1-2 drops of the Low Ionic Strength Saline were added into the IS phase, after which incubation was done in a water bath for about 15 minutes at 37ºC. Low speed of about 500 RPM was applied for centrifugation after shaking the tubes gently.
Use of Gel Card (BioVue) Method to detect antibodies
Gel technology is an example of serological methods that are commonly used nowadays for the detection of the presence of particular antibodies in a sample concerning agglutination reactions. In the case of this essay, a gel (often dextral acrylamide) micro-column which has pores that can allow the passage of free RBCs is impregnated with AHG. However, it is important to note that although the pores in the gel micro-column have the capacity of allowing the passage of free RBCs, they do not allow the passage agglutinated cells. This test used BioVue test cassettes. Also, the microplate walls of the BioVue system ought to bear clear labeling of the red cell antigens used. Then, about 50ul of 0.8 per cent red cells or even 10ul of 3-5 per cent of red cell suspension was added appropriately to different wells.
Secondly, 40ul of test plasma was added to each microplate cell well and the cassettes shook to allow the contents to mix thoroughly and the plates incubated at a temperature range of between 20 and 22ºC for 15 minutes. A BioVue centrifuge system was used to centrifuge the microplate wells for a total of 5 minutes, with 2 minutes at 795 Rate per Minute and 1510 Rate per Minute for 3 minutes. The results were read from the back and the front of the card. Positive results were indicated by the occurrence of agglutination. The occurrence of agglutination forces the formation of a cell layer of the agglutinated cells at the gel micro-column. Often, the layer formed appears at the top of the gel media. In case of negative results, there is no indication of any agglutination and hence all added cells are allowed to pass through the pores of the gel media to settle at the bottom after the process of centrifugation.
Results
This section provides the results of the experiments carried out for the detection of antibodies using various methods such as the Solid Phase Assay, Gel Card Method, and the Tube Method.
Use of Solid Phase Assay to detect antibodies
During the Solid Phase Red Cell Assay procedure, RBC cells were used, which had been tested for anti-K, anti-E and anti-Fya antibodies. The tube and gel (BioVue) methods used the cells to detect the presence of anti-K, anti-D and anti-Fya antibodies. This ensured that the results obtained from all the methods could be compared effectively. Such a comparison was possible because similar antibodies were tested based on same samples and different assays. RBC-coated microplate wells were used in the detection of E antigens, K antigens, JKa antigens, and Fya antigens respectively. The results of the Solid Phase Red Cell Assay are highlighted below.
Anti-Fya antibodies test
The observation of the microplate wells indicated that there were wells in which monolayers did not form as shown in the figure below.

Quality control
- Positive: Formation of monolayers; Presence of anti-Fya antibodies.
- Negative: no formation of monolayers; absence of anti-Fya antibodies.
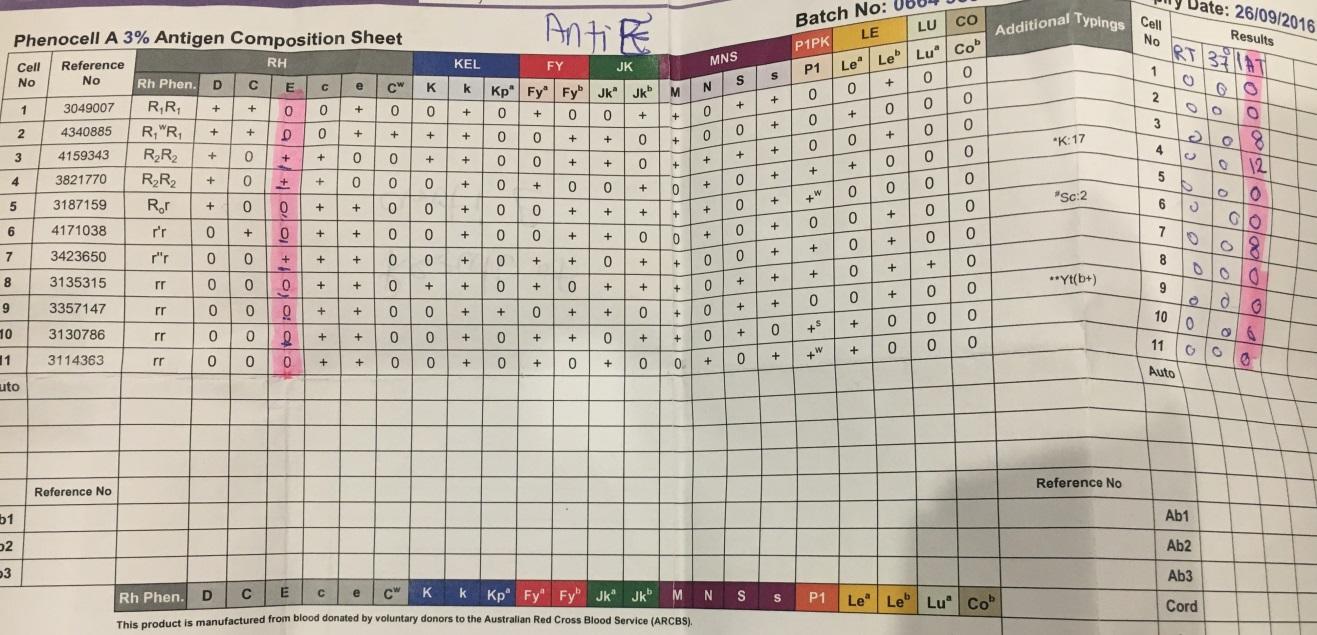
Test for anti-E antigens
The observation of the microplate wells indicated that there were wells in which monolayers did not form as shown in the figure below.

Quality control
- Positive: Formation of monolayers; Presence of anti-E antibodies.
- Negative: no formation of monolayers; absence of anti-E antibodies.
Test for anti-K antigens
The microplate wells indicated that there were wells in which monolayers did not form as shown in the figure below.

Quality control
- Positive: Formation of monolayers: Presence of anti-K antibodies.
- Negative: no formation of monolayers: the absence of anti-K antibodies.
Testing for Monolayer Stability in SPRCA assay
The Solid Phase Red Cell Assay method ends at the point where monolayers of RBC or even buttons of RBCs that are unbound are formed at the bottom of the microplate wells. The endpoints are well-defined and stable such that the test results can be observed at a later date. Despite this, it is important to store the samples under a temperature of 4° C to preserve the RBCs. For this test several microplate wells are used to test for anti-E antibodies. The purpose of the test was to establish whether or not the results from the test were stable. As such, the microplates were stored for about 5 weeks under 4 °C and after investigating their stability it was evident that the results were stable over the test period. The figure below shows the microplate used in monolayer stability in Solid Phase Red Cell Assay.
Testing the sensitivity of the SPRCA assay
The efficiency of any laboratory test in comparison to other methods is very important in the determination of the sensitivity of the concerned laboratory test. Laboratory test sensitivity is described as the quality of a test and its ability to identify correctly all positives without any traces of negatives in any test. Generally, the sensitivity of any given laboratory tests is considered to be its true negative rate. Therefore, the sensitivity of the SPRCA was examined through the use of a papain approach that comprised of test samples and titer of difference concentrations (low and high). The results of the experiment indicated that the dilutions past the 1: 2048 titers were negative with inclusion to all positive samples for anti-Fya antibodies.
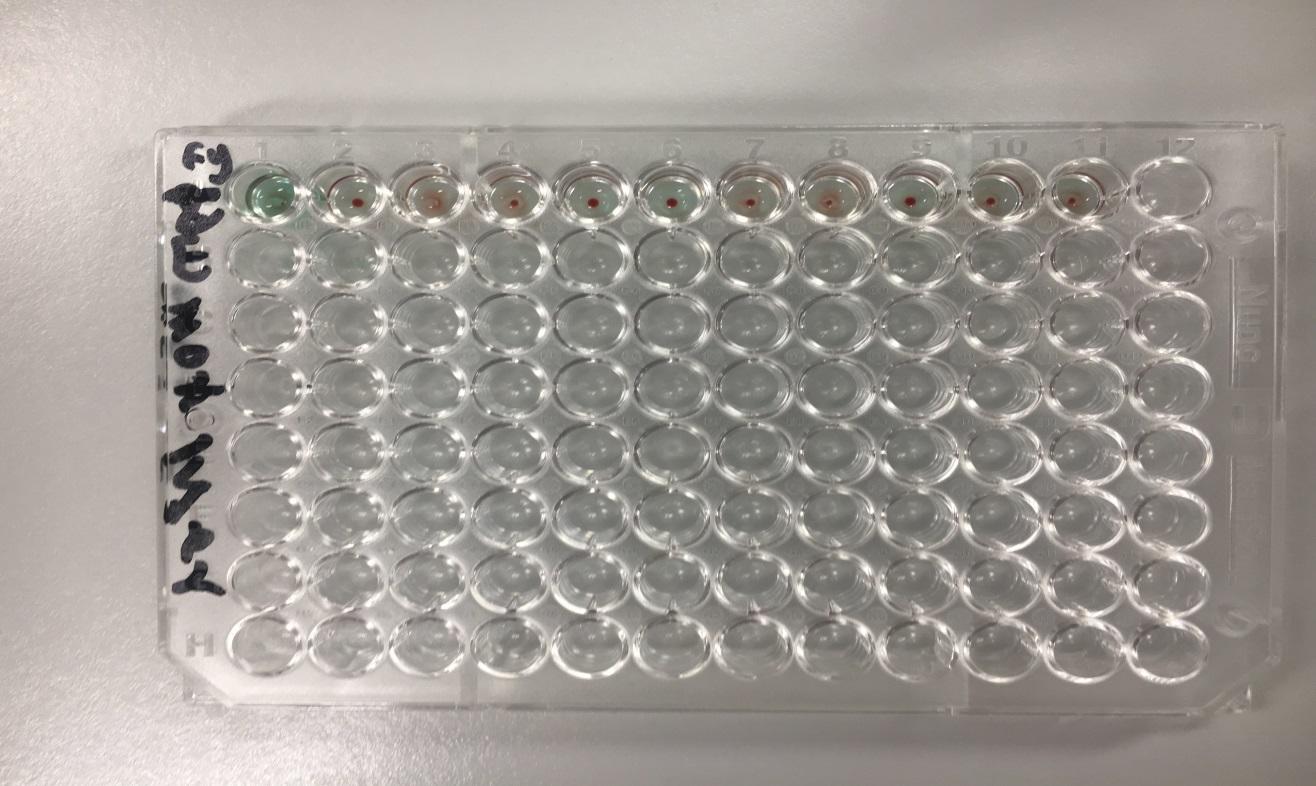
The sensitivity of a test can be described as basically the true positive rate of any given laboratory test. This description implies that a highly sensitive test contains a higher level of certainty that negative tests do not have the disease of the substance tested for. In this case, the sensitivity of the Solid Phase Red Cell Assay method was tested through the use of papain method of the sample serum to 1: 2048, after which the titers were used in the determination of whether or not the assay method was accurate in the determination of all positive as well as negatives despite the low concentrations of antibodies. In the case of this test, normal results were detected for the titer 1: 2048 while nay other titers that were further investigated were found out to be all negative, with the inclusion of the two positive samples. The implication from such results is that the Solid phase assay has the possibility of accurately detecting antibodies in concentrations of 1:2048 or even higher.
Use of Gel Card to detect antibodies
The gel card method takes into consideration the use of BioVue Cassettes that are produced to detect IgG antibodies. A sample was selected for investigation of anti-E, anti-K, and anti-JKa antibodies by using the BioVue system. It was evident that the results from the study aligned with the results from the Solid Phase Red Cells Assay.
Detection of anti-K antibodies through Gel Card test
Positive results through the use of the Gel Card test are indicated by the appearance of a layer that comprises of agglutinated red cells at the top of the gel micro-column. On the other hand, negative results for this test are showed by the presence of delineated red cells sediments at the bottom of the gel micro-columns as evident in the figure below. These results imply that the positive samples have anti-K antibodies, while the negative serum indicates the absence of anti-K antibodies.
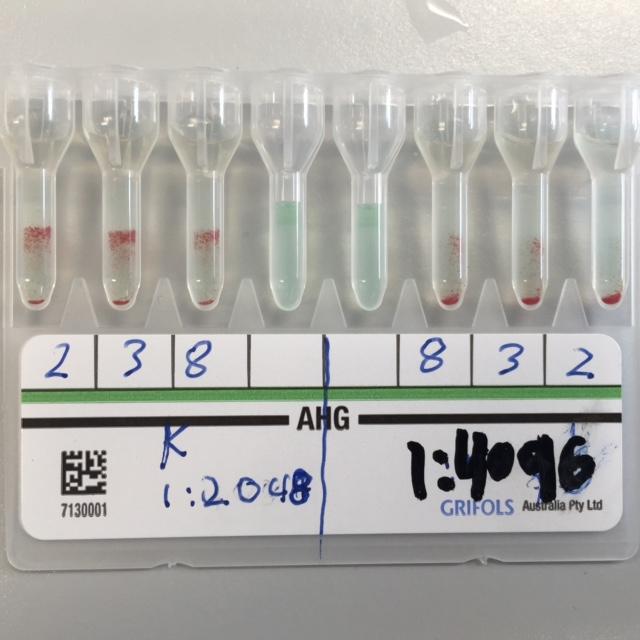
The figure below illustrates the Anti-Jka antibodies Gel Card test.
Gel Card assay sensitivity and specificity
The sensitivity of the Gel Card assay was tested through serial dilutions of already prepared samples. A titer of 1:512 was used to distinguish negatives and positive samples. However, it was found out that all the samples were negative implying that there was a low percentage of antibodies’ concentration in the case of the positive samples. The figure below shows the results of the serial dilution of the Gel Card assay.

Use of Tube Method to detect antibodies
Several phenomenal samples were used to test for the presence of antibodies through the gel card method and the solid phase methods. As evident, the samples comprised of both negative and positive for anti-K antibodies.
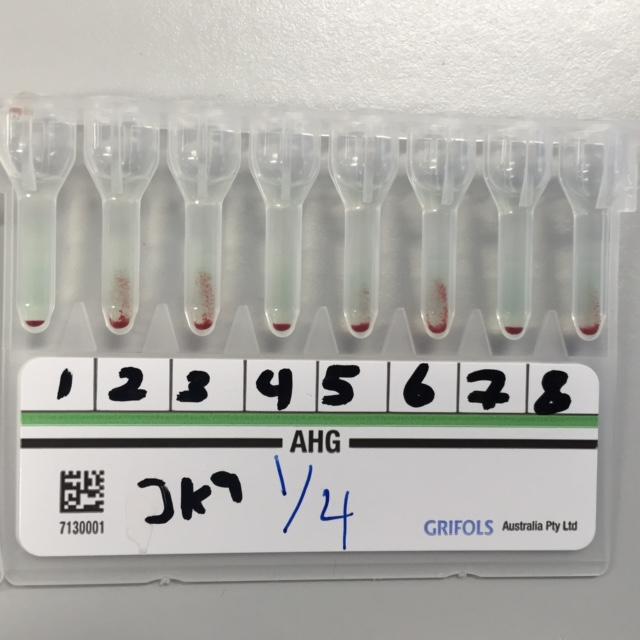
Also, the tube method produced a similar result to the gel card as well as the solid phase assays for anti-Jka in this experiment. In this test, several cell samples were negative for anti-Jka antibodies, while others were positive for anti-Jka. Moreover, it was evident that the reaction strength for the anti-Jka. Such findings aligned with the results obtained in the case of the solid phase and gel card methods. Despite this, the tube method is more advantageous in that it has the capability of indicating the strength of the reaction involving the antibody reaction. The figure below illustrates the results of this study.
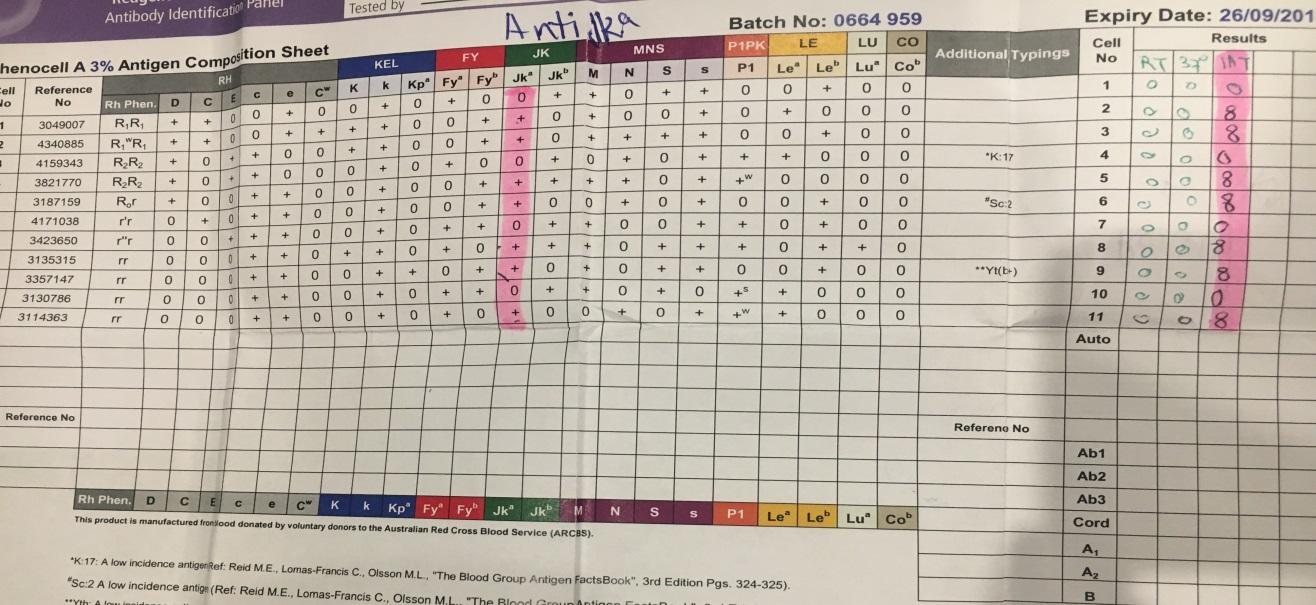
The figure below illustrates the results of the tube test for anti-E antibodies.
Discussion
The primary objective of this study was to carry out a comparative analysis of various serological assays; the tube agglutination, the gel card method as well as the solid phase to determine which one is more efficient in terms of sensitivity and specificity in antigen and antibody detection. This section provides a comprehensive discussion on antigen and antibody detection with a lot of focus on the suitability of various serological tests.
There have been numerous concerns in recent years over the need to ensure that serological tests are highly sensitive and specific. For this reason, advances in technology have led to the introduction of better and fully automated platforms, especially for gel agglutination and the Solid Phase Red Adherence methods. A review of the traditional approach of detection of antibodies reveals that the ability of liquid phase reactions to indicate formed the basis for serological assays. Nevertheless, a lot of change has been witnessed in recent years in terms of improved specificity and sensitivity of blood group serology. For example, a lot of interest has been on the Solid Phase Red Cell Adherence method, which is a tubeless method whose development was aimed at improving the degree with which serological tests are specific as well as sensitive to the antibody under investigation.
In comparison to the traditional approach to antigen and antibody detection, the SPRCA does not rely on agglutination. As such, the method achieves its high sensitivity and specificity by avoiding factors that affect the reliability of serological tests such as the difference in the ration of cells used in the test to serum, the amount of time the reagents are under incubation, the ionic strength, as well as their pH values. In most of cases, achieving standardization and control of the tests can be challenging due to the above factors. On the other hand, concerns have been raised over the use of numerous variables that tend to reduce the specificity, sensitivity and stability of agglutination tests.
Modern technology used in the detection of antigens and antibodies makes the use of microplates. Such technology has greatly changed the approaches to serological tests by allowing the improvement of tests’ specificity and sensitivity. Nowadays, solid phase and the gel column technology are adversely used to detect various types of antibodies ranging from anti-E, D, Fya, and K to anti-Jka. Despite the fact that these two approaches are significant in the field of medicine, they show differences in terms of the cost involved, the stability of results, and specificity and sensitivity to endpoint stability, as well as sensitivity and specificity.
With respect to the objective of this experiment, traditional tube agglutination test, the solid phase assay, and the gel card method were analyzed through a series of tests aimed at establishing their specificity, sensitivity as well as the stability of their endpoints. Blood group antigens were used in this experiment based on their differences such as occurrences and antigenicity.
A review of the experimental results shows that the three tests had high level of similarity in terms of detecting various antigens and antibodies such as anti-K, anti-E, and anti-Fya and anti-Jka when assessed against RBC cells. The similarity in the results of antigen and antibody detection using the three tests is an indication of the reliability of the tube agglutination, the gel card method as well as the solid phase approach to antibody and antigen detection. However, it was noted the gel card method and the solid phase assays had high level of sensitivity and specificity in the detection of antigens and antibodies in comparison to the tube agglutination approach. Based on these findings, it suffices that the solid phase, the gel card and the tube assays can be used as a background for serological testing especially in the transfusion sector. Nevertheless, it is important to put into consideration other factors that might affect the suitability of these methods such as the stability of the endpoints and their standardization.
Stability
The stability of the endpoints of the serological essays was examined through observation of the monolayer formed over several weeks under a temperature of 4ºC. It was observed that the monolayers formed through SPRCA and gel card method were stable over the experiment period. In spite of this, the solid phase approach was highly stable in comparison to the gel card and the tube methods. The implication is that the solid phase method is more reliable than the gel card especially where results are needed for later reference.
Specificity and Sensitivity
Papain test was used alongside anti-Fya with anti-E to investigate the sensitivity of the tests. The results of the experiment indicated that the solid phase test is highly sensitive and specific in comparison to the other two tests. In addition, the gel card method had a high sensitivity too. Such results were in line with the findings of The American Food and Drug Administration (FDA) agency on its comprehensive analysis of the solid phase and gel card assays before approving their use. Based on the findings of FDA, the gel card and SPRCA methods are used in blood banks as well as in clinical laboratories in many parts of the world due to the fact that they have a high specificity and sensitivity level. In spite of this, the study found out that the gel card method was not as sensitive as the SPRCA and thus, cannot be used in the detection of antibodies of low concentration. The implication from such findings is that the detection of antigens and antibodies is highly depended on their concentration. Therefore, lowly concentrated antibodies are effectively detected through the use of the solid phase method.
Conclusions
Based on the experimental analysis of the three types of serological tests, it was evident that the solid phase and the gel card technologies have stable endpoints for any tests implying that the results from such tests can be used at a later date. Secondly, it was evident that the solid phase assay makes the use of microplates, indicator cells, test sera, as well as anti-human globulins for the purpose of facilitating the antibody-antigen reactions which led to monolayer formation in the case of positive samples. Contrary to the solid phase method, the gel card technology, takes into consideration the application of gel micro-columns that are used for the addition of the sera and test cells for incubation, as well as observation to establish the occurrence of agglutination.
According to the results of the experiment and the various tests, it can be concluded that both the gel card and the solid phase methods make use of standard assay reactants as well as procedures hence, enabling the elimination of several processes like the tube-shaking technique that are highly subjective. Similarly, the advancement in the antigen and antibody detection technology through the use of gel card and solid phase methods have led to high stability, sensitivity and specificity in terms of results and tests, making it possible to interpret the results based on standard approaches. For this reason, the SPRCA and gel card methods are suitable in the differentiation of positive and negative samples as opposed in the case of tube agglutination method. Moreover, these methods have a high sensitivity and specificity making them appropriate for use in sensitive areas such as in blood banks.
References List
Ching E. Solid Phase Red Cell Adherence Assay: A tubeless method for pretransfusion testing and other applications in transfusion science. Transfusion and Apheresis Science. 2012; 46(3):287-291.
Duguid J K M, Bromilov I M. New technology in hospital blood banking. Journal of Clinical Pathology. 1993; 46(7): 585-8.
Knight R C, de Silva, M. New technologies for red-cell serology. Blood Reviews. 1996; 10(2): 101-110.
Plapp F V, Sinor L T, Rachel J M, Beck ML, Films M I, Coenen W M, et al. A solid-phase antibody screen. Brief Scientific Reports 1984; 82(6): 719-21.
Lee, M. (2013). Basic skills in interpreting laboratory data. London: ASHP.
Knight R C, de Silva, M. New technologies for red-cell serology. Blood Reviews. 1996; 10(2): 101-110.
Weisbach V, Kohnhauser T, Zimmermann R, Ringwald J, Strasser E, Zingsem J, Eckstein R. Comparison of the performance of microtube column systems and solid-phase systems and the tube low-ionic-strength solution additive indirect antiglobulin test in the detection of red cell alloantibodies. Transfusion Medicine. 2006; 16: 276-284.
Adriaansen M, Perry H. Validation of column agglutination technology for blood group alloantibody titration. New Zealand Journal of Medical Laboratory Science. 2013; 3: 92-96.
Summers T, Johnson V, Stephan JP, Johnson G J, Leonard G. The value of automated gel column agglutination technology in the identification of true inherent D blood types in massively transfused patients. Transfusion. 2009; 49(6): 1672-1677.
Bunker ML, Thomas C L, Geyer S J. Optimizing pretransfusion antibody detection and identification: a parallel, blinded comparison of tube PEG, solid-phase, and automated methods. Immunohematology. 2001; 41(10): 621-626.
Fernanda Morelati M. New technologies in immunohematology. Blood Transfusion. 2007; 5(2):58. Web.
Janeway CA Jr, Travers P, Walport M, et al. Immunobiology: The Immune System in Health and Disease. 2001. 5th Edn. Garland Science
Ching E. Solid Phase Red Cell Adherence Assay: A tubeless method for pretransfusion testing and other applications in transfusion science. Transfusion and Apheresis Science.2012; 46(3):287-291.
Weisbach V, Kohnhauser T, Zimmermann R, Ringwald J, Strasser E, Zingsem J, Eckstein R. Comparison of the performance of microtube column systems and solid-phase systems and the tube low-ionic-strength solution additive indirect antiglobulin test in the detection of red cell alloantibodies. Transfusion Medicine. 2006; 16: 276-284.
Lin, J.S, Lyou J.Y, Chen Y.J, Chen P.S, Liu H.M, Ho C.H, Hao T.C, Tzeng C.H. Screening for platelet antibodies in adult idiopathic thrombocytopenic purpura: A comparative study using solid-phase red cell adherence assay and flow cytometry Chao-Hung Chin Med Assoc, 2006 ;69 (12).
Salama OS, Aladl DA, Ghannam D M.El, Elderiny WE. Evaluation of platelet crossmatching in the management of patients refractory to platelet transfusions. Blood Transfus. 2014; 12(2): 187–194.
Simon, T. L., Snyder,. L., & Stowell, C. P. (2011). Rossi’s principles of transfusion medicine. New York, NY: John Wiley & Sons.
Schlaf G, Pollok-Kopp B and Altermann W. W. Solid phase-based cross-matching to Avoid kidney allografting against Donor-Specific anti-HLA antibodies: Long Term Experience with a Procedure Allowing Highly Reliable Diagnoses. Austin J Nephrol Hypertens. 2014;1(6): 1028.
Pinkerton PH, Chan R, Ward J, Coovadia AS. Sensitivity of column agglutination technology in detecting unexpected red cell antibodies. Transfusion Medicine 2008; 3(4):275 – 279.
Salama OS, Aladl DA, Ghannam D M.El, Elderiny WE. Evaluation of platelet crossmatching in the management of patients refractory to platelet transfusions. Blood Transfus. 2014; 12(2): 187–194.
Ajpai, M., Kaur, R., & Gupta, E. (2012). Automation in immunohematology. Asian Journal of Transfusion Science, 6(2), 140-44.
Wallach, B. (2007). Interpretation of diagnostic tests. New York, NY: Lippincott Williams and Wilkins.
Wild. D. The Immunoassay Handbook 2005. Elsevier pg. 33-35.
Lin, J.S, Lyou J.Y, Chen Y.J, Chen P.S, Liu H.M, Ho C.H, Hao T.C, Tzeng C.H. Screening for platelet antibodies in adult idiopathic thrombocytopenic purpura: A comparative study using solid-phase red cell adherence assay and flow cytometry Chao-Hung Chin Med Assoc, 2006 ;69 (12).
Matsuura. E, Igarashi. Y, Yasuda. T, Triplett. D.A and Koike T. Anticardiolipin antibodies recognize beta -2-glycoprotein I structure altered by interacting with oxygen modified solid phase surface. JEM 1994; 179 (2): 457.
Gebel M, Bray R. A, HLA antibody detection with solid phase assays: great expectations or expectations too great? Am J Transplant. 2014; 14(9):1964-75.
Mahmood T, Yang P-C. Western Blot: Technique, Theory, and Trouble Shooting. North American Journal of Medical Sciences. 2012;4(9):429-434. Web.
Finck, R.H, Davis,R.J Teng, S, Goldfinger. D, Ziman,A.F, Lu Q and Yuan S. Performance of an automated solid-phase red cell adherence system compared with that of a manual gel microcolumn assay for the identification of antibodies eluted from red blood cells Immunohematology 2011;27( 1).
Simon, T. L., Snyder,. L., & Stowell, C. P. (2011). Rossi’s principles of transfusion medicine. New York, NY: John Wiley & Sons.
Reverberi R and Reverberi L. Factors affecting the antigen-antibody reaction. Blood Transfus. 2007; 5(4): 227–240.
Hammering, D. (2012). Modern blood banking and transfusion practices. Philadelphia, PA: FA Davis Company.
Schlaf G, Pollok-Kopp B and Altermann W. W. Solid phase-based cross-matching in order to Avoid kidney allografting against Donor-Specific anti-HLA antibodies: Long Term Experience with a Procedure Allowing Highly Reliable Diagnoses. Austin J Nephrol Hypertens. 2014; 1(6): 1028.
Screening Donated Blood for Transfusion-Transmissible Infections: Recommendations.Geneva: World Health Organization. 2009.
Ching E. Solid Phase Red Cell Adherence Assay: A tubeless method for pretransfusion testing and other applications in transfusion science. Transfusion and Apheresis Science 2012; 46(3):287-291.
Pinkerton PH, Chan R, Ward J, Coovadia AS. Sensitivity of column agglutination technology in detecting unexpected red cell antibodies. Transfusion Medicine 2008; 3(4):275 – 279.