Introduction
Some of the most incredible forms that are present in nature and have been artificially replicated by man are liquid crystals. The amazingness of this phenomenon is due primarily to their nature — liquid crystals are not solids or liquids but instead represent a unique, transitional state between these phases. Accordingly, liquid crystals are characterized by the properties of both liquids, that is, fluidity, and crystals, that is, anisotropy. This equilibrium state, however, is not entirely stable since the action of external factors, whether temperature or electromagnetic forces, can lead to the transformation of liquid crystals into only solid or, on the contrary, only liquid phase. Such properties of liquid crystals are fully justified by the presence of molecules of a particular type, namely elongated disc-shaped molecules that can be oriented in space in such a way that their physical and chemical properties change. In particular, under the action of electric field forces, the elongated molecules align themselves in such a way as to minimize the effects of the fields and save their own internal energy. In other words, liquid crystals can be influenced by electric fields to change their behavior, a phenomenon that underlies the widespread use of liquid crystalline materials in the industry. Thus, this report examines and reviews in detail the fundamental properties of liquid crystals, discussing their nature and the effects of external factors. In addition, a brief overview of the history of liquid crystals discovery is offered. Toward the end of the paper, the industrial use of liquid crystals is discussed.
A Brief History of Discovery
The study of cholesterol compounds was a predictor of the discovery of the structure of liquid crystals. In the late 19th century, in 1888, Austrian botanist and chemist Friedrich Reinitzer, in an attempt to establish the exact geometry and properties of a polycyclic lipophilic alcohol-based substance, accidentally discovered that the object under study appeared to have two melting points (DiLisi, 2019). It is worth recalling that the melting point is a quantitative physical quantity that equals the temperature value at which a substance changes from a crystalline state to a liquid state (Qiu, 2018). Accordingly, the higher the melting point of a particular substance, the more thermal energy it needs to impart in order for it to reach the critical threshold for a phase transition. Perhaps the best-known melting point is that of solid ice at 0°C — 32 °F or 273 K — but there are different melting points for each of the known bodies. For an unknown substance based on cholesterol molecules, cholesteryl benzoate, Reinitzer found that it had two melting points at once. More specifically, at about 146°C, the solid became a cloudy, viscous liquid that only made the transition to a liquid, transparent state when it reached 179°C (DiLisi, 2019). This meant that there was some transition stage for the substance with a rather large interval of temperature existence, which corresponds to stability. The confirmation of the discovery of the new state was made possible by the cooperation of Reinitzer with Otto Lehmann, a German physicist in the direction of crystal optics. Thus, the existence of liquid crystals was discovered, which combined the properties of both liquid substances and solid crystals.
However, as is often the case in academia, any bold statement was severely criticized by the scientific community. The idea of new forms of liquid crystal matter was not immediately supported (DiLisi, 2019). Among the key counterarguments, academics hypothesized an ordinal mixture of two substances of different phases, although the ancestors of liquid crystal physics repeatedly proved their purity. Over time, researchers accepted the idea, as increasingly scientific discoveries confirmed the existence of liquid crystals. In addition, new discoveries were made in this field, which put difficult pressure on the opponents of this theory.
Packing of Molecules as the Basis of Properties
Before Reinitzer’s and Otto’s theories, it was believed that matter had only three stationary forms and, accordingly, two transition points. The transformation from solid to liquid had a melting point, while the transition from liquid to gaseous had a boiling point. However, these views were radically revised with the theory of liquid crystals, which postulated that there are many more states than three. Such diversity could only be possible in many ways due to the unique nature of the molecules that form liquid crystals. An essential condition for the transition of substances to the state of liquid crystals that is for the potential for the existence of such materials is the presence of an asymmetric form of the molecules. The shape of such particles usually represents elongated plates, cylinders, or other similar geometries, which contribute to the active joint alignment of the molecules under the action of external factors, whether temperature or electric field (Kato et al., 2018). In this sense, it is worth highlighting separately that liquid crystal molecules were initially thought to be rod-shaped. However, over time, science evolved, and it became clear that in addition to rod-like particles, the basis of liquid crystals can be represented by more complicated complexes: disks, plates, or stacks. However, it is clear that each of the general types of particle structure can be represented by different configurations. In order to use a rigorous classification and facilitate effective communication between researchers from different countries, it was decided to develop a general system for the structure of molecules that form liquid crystals. This includes such forms as smectic, nematic, and cholesteric type structures.
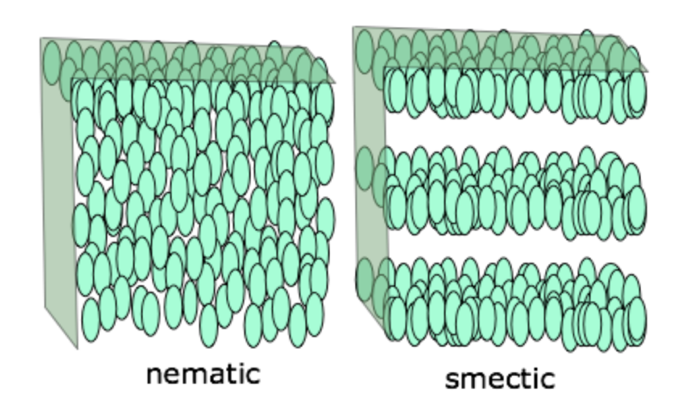
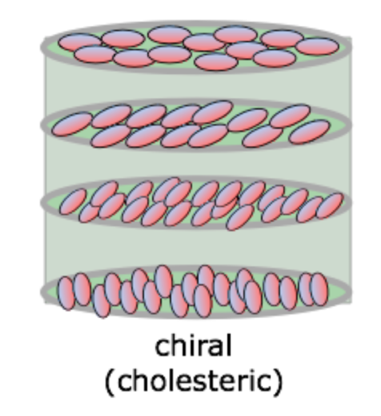
Figure 1 describes a schematic representation of each of the three types of structures. The first of them, nematic, is represented by the arrangement of molecules in which the long axes are oriented in one direction – this is an ordered arrangement of particles. The common vector along which all particle axes are directed is called the nematic director (Walton et al., 2018). In this state, the disclinations are perfectly observable visually when the crystal is illuminated by natural light: in these zones, the orientation of the elongated molecules changes radically. This is remarkable because such regions with different axis orientations form different, sometimes even opposite, domains. There is a change in the refractive index of light on the disclinations, which is the reason for the cloudy appearance of such crystals.
The second structure in Figure 1 shows the smectic type of arrangement of molecules, which literally stands for soap or soap-like. This name for smectic liquid crystals is not coincidental: these crystals were initially isolated from soap (Tyagi, 2018). It is noticeable that, in this configuration, the ends of the particles seem to be fixed in invisible planes parallel to each other. The central axis of each particle appears to be perpendicular to the plane in which the molecules are located. In fact, at each level of this configuration, the molecules are strictly oriented relative to each other due to the forces of disperse interaction. Of the three types of configurations, the smectic is the closest to actual crystalline substances. Finally, the third type of arrangement is cholesteric, or one of the most complex. In this way, the molecules are more like plates, which are arranged in parallel planes. The orientation of the axes is different in all layers because the overall structure is twisted. For this model, there are specific geometric characteristics that define the shape of a molecule: angle of a helix, number of its twists, the distance between two points located in one plane, and step of a cholesteric helix. If one considers this structure more generally, one can see that nematic crystals are generic forms for cholesteric crystals, which the spiral director explains well in Figure 2.
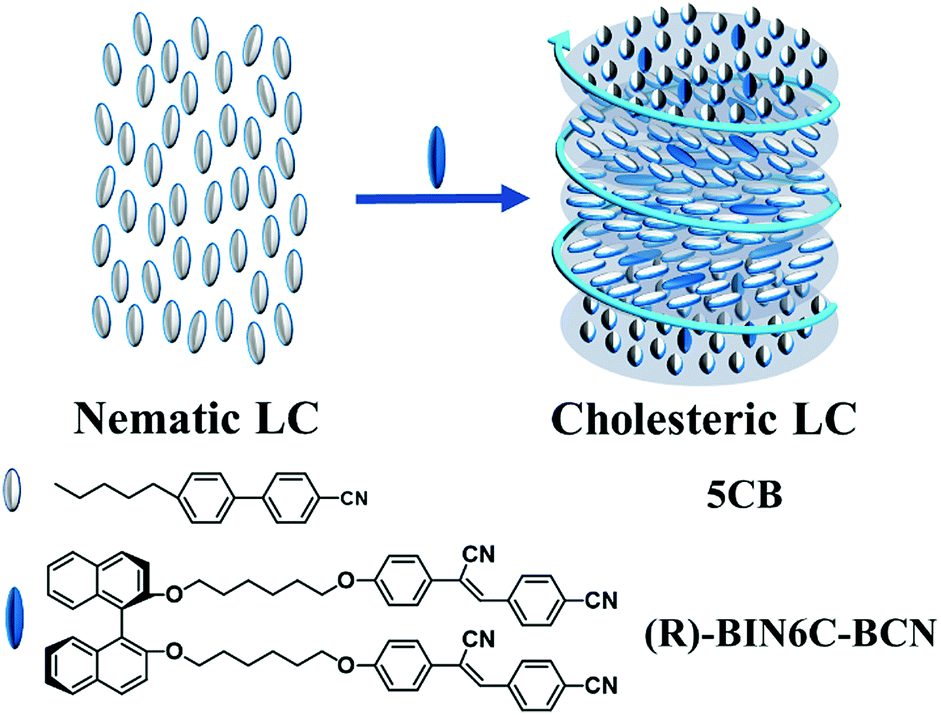
Smectic, nematic, and cholesteric liquid crystals form a joint group called thermotropic. Thermotropic crystals are classical materials in the usual sense that are formed by heating solids and can only exist within certain temperatures, as was the case with the substance discovered by Reinitzer (DiLisi, 2019). There is sufficient evidence showing that the possibility of the existence of such materials is realized only if the substituents in the aromatic base molecules are located in the para-positions (Sparavigna, 2019). However, there is another group of crystals called lyotropic materials. Such crystals are formed only in liquid media as a result of the formation of supramolecular aggregates, whereas a rule polar solvents act as the second component. These mixtures contain both hydrophobic and hydrophilic parts, so lyotropic crystals are generally amphiphilic.
Properties of Liquid Crystals
In turn, a particular particle alignment design allows liquid crystals to have specific properties, whether optical, physicochemical, or mechanical. In other words, the properties of the substance depend on the direction of the molecules triggered by external factors. The same unique form determines the fact that, on the one hand, liquid crystals are liquids with high fluidity in the vessels in which they are found, but on the other hand, their viscosity resembles the consistency of liquid glue. It is important to emphasize that the substance in the state of liquid crystals has high stability, which means that they may well be considered the fourth form of existence of matter, liquids, gases, and solids. The fact of the transition state, moreover, justifies the name of such a mesomorphic phase. However, it should be understood that mesophase is a broader concept, so any liquid crystal is a mesophase, but not any mesophase is a liquid crystal.
The structure of liquid crystal materials directly affects their properties in terms of the packing of particles in space and their orientation. One of the most apparent influences of structure is noticeable for cholesteric liquid crystals. Because of the curvature along the vertical helix shown in Figure 2, such materials can selectively reflect the light striking them. In particular, the incidence of light at a fixed angle causes reflection of only a particular wavelength — this depends on the structure of the crystal in question, the degree of its twist, and the incident light — resulting in the liquid crystal becoming colored in only one shade. The reflected wavelength may be located not in the region of visible light for the electromagnetic spectrum, but in the regions of ultraviolet or even infrared radiation, which will not be perceived by the human eye. In this case, the crystal may appear as a turbid substance, taking color under the influence of ultraviolet or infrared lamps, respectively. At the same time, it is necessary to remember the properties of anisotropy, which are characteristic of cholesteric crystals. The angles of refraction of the incident light and the speed of its propagation in the medium are functions determined by the geometry of a particular crystal face. This means that when the observation angle changes, the visual characteristics of the crystal also change: in particular, changes in the coloration of the material are observed, which qualitatively distinguishes colored crystals from pigments that have a fixed coloration regardless of the angle of incidence of light.
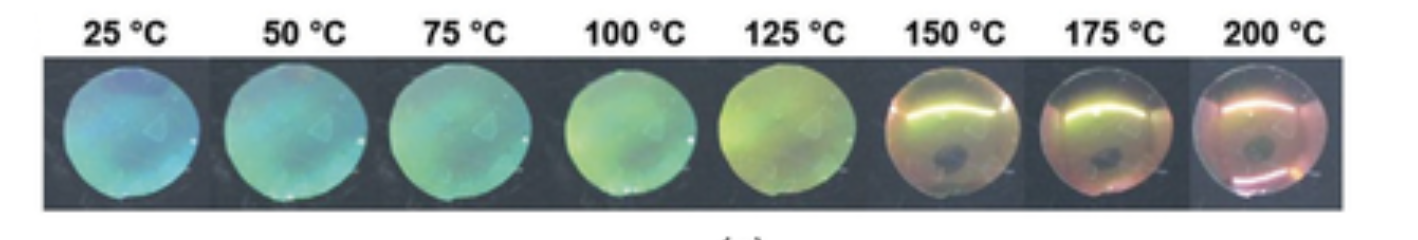
The balance of the structure inside the liquid crystals is unstable and is relatively easily disrupted by external forces. One of the easiest ways to displace this stability is temperature, the action of which can modify the aggregate state of the substance. It is worth recalling that the originally discovered liquid crystal, a cholesterol derivative, had an interval of temperature existence of about thirty degrees. During gradual heating, more thermal energy is communicated to the particles, as a result of which the degree of their retention relative to each other decreases: the particles tend to move away. Because of this structural change effect, a change in color is, in turn, observed (DiLisi, 2019). Figure 3 perfectly captures this trend, where an increase in ambient temperature decreases the wavelength of the reflected light, leading to a persistent violet coloration. Moreover, the phenomenon of the transition state determines the possibility of such materials turning into a liquid state when the critical melting point is reached or changing into a crystalline state when the temperature of the environment decreases.
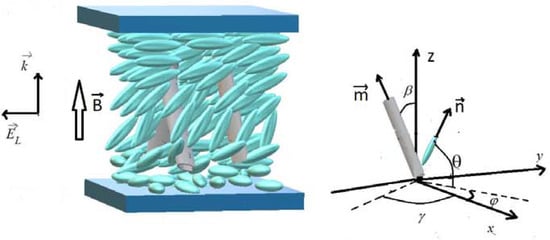
Not only temperature but also the electric field is an active factor that affects the structure and properties of liquid crystal materials. Generally speaking, all liquid crystals are dielectrics because they do not have free-charged particles within the matrix – but the affinity with solids, expressed in anisotropy, gives rise to some of the electrical properties of crystals. When electricity is applied, the particles of matter are ordered according to the external field strength vector, and this effect is strictly dependent on the orientation of the object in space. However, liquid crystals used in industry are always confined to some frame, for example, the glass of a vessel containing a viscous liquid. The action of the electric field causes a change in the principal axis directors of each molecule, creating a Fréedericksz transition (Cirtoaje et al., 2020). Figure 4 perfectly shows the essence of this effect: due to the electric field, the principal axes are inhomogeneously directed, but the particle axes are perpendicular to the field vector closer to the vessel’s walls. This phenomenon is of high importance for industrial use of liquid crystals because manipulating the electric current-voltage changes their ability to transmit polarized light.
Use of Liquid Crystals in Industry
As it was shown, materials based on liquid crystals can be used in light of their ability to change light absorption by manipulating the voltage of the electromagnetic field. As it is known, when light propagates, photons oscillate in different directions in a plane perpendicular to the plane of motion of the light beam: this effect is the polarization of light (Stroski, 2017). Polarizers allow the oscillation of photons to be unified in one direction, which is filtered by the polarization plate matrix.
The polarization plates are what are the edge layers in the electro-optical cell so that the liquid crystal material, modified to the required regulation, is poured inside. The director must be arranged nematic so that the axis of each particle appears perpendicular to each polarization plate, as shown in Figure 5. When these conditions are met, the beam of light passes entirely through the electro-optical cell, not that it creates total transmittance: as a result, the display is black. Applying an external electromagnetic field to this cell, however, causes the director to change. In this case, the axes of the particles will be reoriented so that they become parallel to the polarization planes, but not perpendicular. Now the light passing through the cell does not pass through entirely but repeats the spiral rotation; as a result, even if there are two polarization plates perpendicular to each other, the light is not absorbed. In other words, the maximum dielectric permittivity tends to be in the same direction as the intensity vector of the electromagnetic field applied to the cell. This effect realizes its potential in a matrix consisting of many liquid crystal squares, which allow creating a pixel picture.
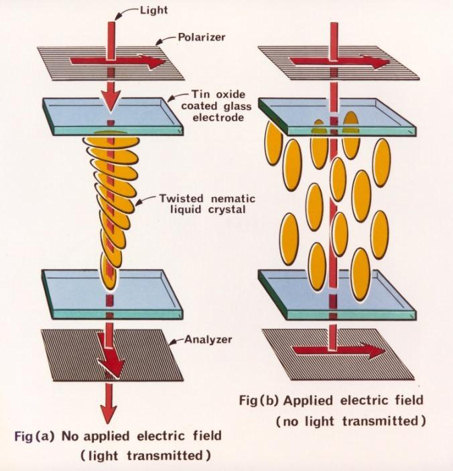
Liquid crystal displays have several advantages for their use in electrical engineering. First, such materials have a high capacity for self-organization, but the stability of their director is impermanent and can be altered by external factors. Second, a viscous fluid can easily fill small spaces, which means that the electro-optic cell can be tiny in potential. Third, they do not require large voltages to control their operation, so liquid crystal displays are used even on handheld smartwatches. Fourth, the effect of light transmission and reflection, augmented by placing a mirror closer to the lower electrode in the cell, allows for high contrast images. Fifth, which is already common practice, by increasing the number of electrodes and changing the cell’s shape, unique form-factors of liquid crystal screens of TVs, computers, smartphones, and other electrical equipment can be created.
Conclusion
In conclusion, it is worth emphasizing that the critical feature of liquid crystal materials — in other words, combining the anisotropic properties of solids with the fluidity of liquids — presents them with great potential for use in electrical engineering. Liquid metals are highly organized systems consisting of molecules. Depending on the packing of molecules, nematic, smectic, and cholesteric liquid crystals are distinguished. In contrast, lyotropic liquid crystals, which are formed by two or more components, are distinguished. The ability of liquid crystals to react actively to external factors, whether temperature or electromagnetic field, allows them to be used as the primary material for modern displays.
References
Cirtoaje, C., Iacobescu, G., & Petrescu, E. (2020). Freedericksz transitions in twisted ferronematics subjected to magnetic and laser field. Crystals, 10(7), 1-13.
DiLisi, G. A. (2019). An introduction to liquid crystals. Morgan & Claypool Publishers.
Kato, T., Uchida, J., Ichikawa, T., & Sakamoto, T. (2018). Functional liquid crystals towards the next generation of materials. Angewandte Chemie International Edition, 57(16), 4355-4371.
Lu, P., Chen, Y., Chen, Z., Yuan, Y., & Zhang, H. (2021). Electric field, temperature and light-triggered triple dynamic circularly polarized luminescence switching in fluorescent cholesteric liquid crystals with a large dissymmetry factor. Journal of Materials Chemistry C, 9(20), 6589-6596.
Lower, S. (2020).Liquid crystals. Chemisty LibreTexts.
Sparavigna, A. C. (2019). The earliest researches on liquid crystals [PDF document].
Stroski, P. N. (2017). Liquid crystal display. EL.
Tyagi, Y. (2018). Liquid crystals: An approach to different state of matter. The Pharma Innovation, 7(5, Part H), 540-545.
Qiu, F. (2018). Practical considerations. In F. Qiu and G. Scrivens (Eds.), Accelerated Predictive Stability (pp. 75-103). Academic Press.
Walton, J., Mottram, N. J., & McKay, G. (2018). Nematic liquid crystal director structures in rectangular regions. Physical Review E, 97(2), 1-12.
Zhang, W., Froyen, A. A., Schenning, A. P., Zhou, G., Debije, M. G., & de Haan, L. T. (2021). Temperature‐responsive photonic devices based on cholesteric liquid crystals. Advanced Photonics Research, 2(7), 1-27.