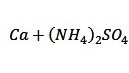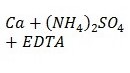Introduction
Analytical chemistry techniques include, but are not limited to, the study of the spectroscopic properties of materials in light of added loading, whether it be electromagnetic radiation, directed light or performing a chemical reaction whose purpose is to facilitate observation of the elementary structure of matter. Atomic absorption spectroscopy, AAS, is one such method of investigation that allows the qualitative identification of most of the chemical elements of the Periodic Table using the atomic absorption spectra of the combustion products of the parent substance. It should be clarified that the atomic absorption method of investigation is based on the absorption of radiation by unbound atoms, and therefore, since free atoms and multi-atomic particles give unique lines in the spectrum, the most important prerequisite of AAS is the conversion of the substance to atomic pair. The translation of the substance from the atomic to the first excited ionic state is realized through the use of an open flame in the atomizer: accordingly, the more concentrated the solution was initially used, the more light energy will be absorbed. At the same time, there are a number of external factors that make it possible to control the intensity of this transition. This thought was the basis of this laboratory work, the purpose of which was to study the chemical and physical interferences for Calcium atomization and some of the techniques for overcoming them.
Results and Discussion
The study’s key results were to obtain spectroscopic characteristics for nine working calcium solutions, a mixture of calcium and ammonium sulfate, and this mixture with the addition of EDTA solution. Each of the samples was excited with an air-acetylene burner, and the products of chemical combustion were recorded with an analytical signal through exposure to radiation. The outputs for the working solutions are shown in Table 1.
Table 1. Interference Solutions for Calcium, Calcium-Sulfate Mixture, and Calcium-Sulfate-EDTA Mixture Data.
Based on this table, it is possible to conclude a specific pattern. In particular, it is easy to see the tendency for the occurrence of noise and interference in the signal registration when additional impurities are added to the solution. Thus, the addition of ammonium sulfate decreases the average absorbance, while further addition from EDTA solution increases the average relative to the original one. In addition, the analytical signal was measured for two series of calcium solutions and a mixture of calcium and potassium chloride depending on the amount of added substance, but acetylene and nitrous oxide (I) flame were used for these measurements. It is known that this burner can provide a much higher temperature due to the thermodynamic instability of nitrous oxide, which means that more additional energy is expended for atomization, and increased transparency, suitable for more uninterrupted signal registration. The results of signal measurements for solutions of series A and B are given in Table 2.
Table 2. Measurement Results for Two Series of Ionization Interference Solutions
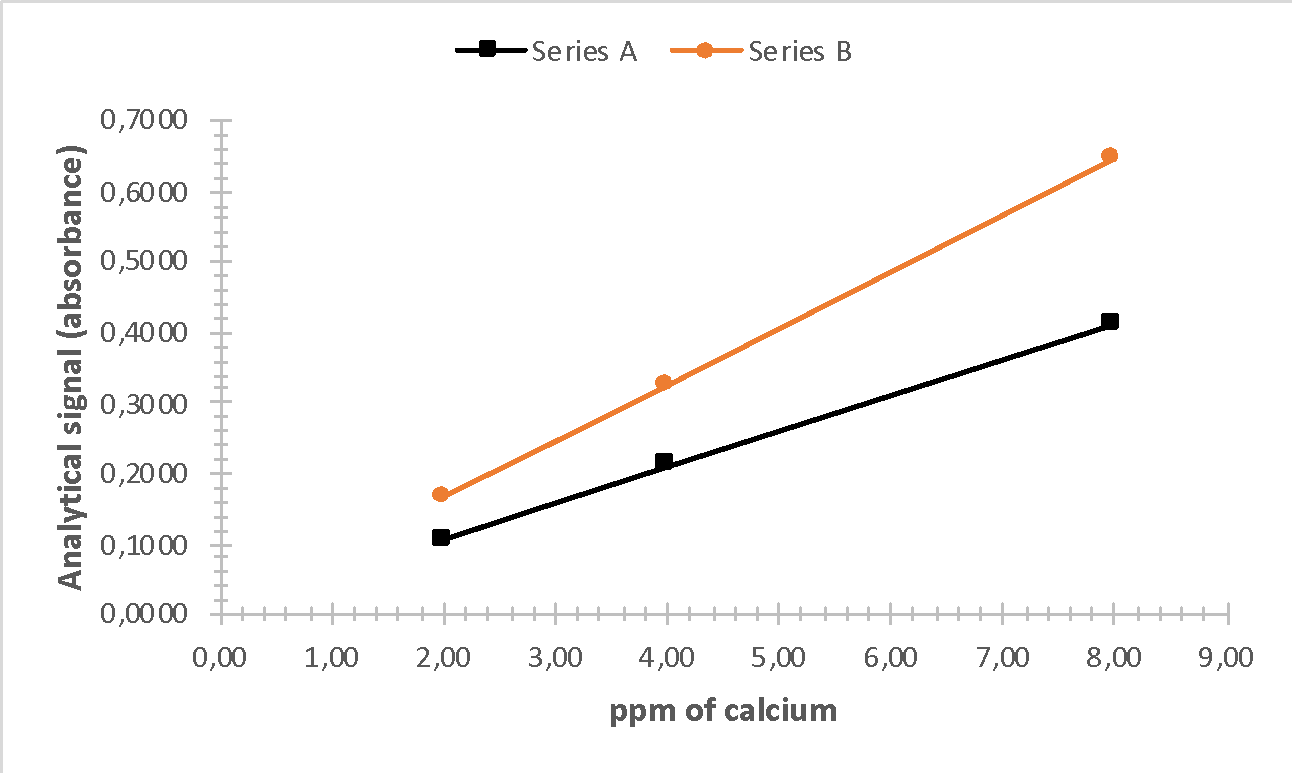
Even a cursory analysis of Table 2 can show that there is a linear relationship between the output signal and the concentration of the initial substance (calcium) in the solutions. This idea was used to plot the absorbance vs. calcium concentration in ppm (Figure 2) for both series. Based on this figure, it is pertinent to note that with equal calcium content, the analytical signal of the impurity solution was higher. This effect can be explained by the presence of additional atoms (potassium), which also undergo atomization and are detected by the recorder.
Reference
Walker., S.; Stevenson., B.; Peterson, J.; Donati, G. L. Determining Micro- and Macro-Elements by Flame Atomic Absorption Spectrometry. Chem Educat 2016, 21, 264-272.