Introduction
Obesity is one of the most significant public health problems in the world. It damages physical and mental health, thereby leading to the development of chronic diseases and comorbidities that require lifelong treatment. In 2017, the number of obese people in China ranked first in the world for the first time, at 89.6 million or about 100 million people. In 2016, the American Association of Clinical Endocrinology (AACE) proposed reclassifying obesity as a chronic disease based on obesity, to reflect the scope of health complications associated with this condition.
Today, effective weight control can alleviate or even reverse related chronic diseases, such as diabetes, hypertension, hyperlipidemia, and gastroesophageal reflux, depending on how one manages the problem. According to the 2016 Chinese Overweight/Obesity Medical Nutrition Therapy Expert Consensus, effective and safe weight loss is based on a combination of diet, exercise, psychotherapy, behavioral intervention, healthy education, lifestyle changes, stratified management decisions, and drug selection according to indications, as well as surgical treatment. Among them, diet control is the key to achieving successful weight loss. Expert consensus recommends three effective diet patterns: calorie-restricted diet (CRD), high-protein diet HPD, and intermittent fasting4.
The present investigation will compare the maintenance of muscle mass by the three weight-loss diets highlighted above. The findings highlighted above exemplify the risk that dietary interventions could lead to the creation of non-fat body mass. At the same time, maintaining muscle mass is crucial for preventing fatigue and weakness caused by weight loss, as well as for maintaining the basal metabolic rate of associated enzymes. At the same time, it can more effectively alleviate and reverse chronic metabolic diseases. Therefore, this study aims to identify an optimal diet pattern for weight loss by examining the changes in body composition of patients who have lost weight using the aforementioned three diet patterns.
Materials and Methods
This study aims to explain the effects of muscle loss and SMI on obesity treatment and management. Consistent with this line of probe, the primary objective of the investigation is to determine the effects of high-protein diets on muscle and fat mass. This chapter highlights the techniques the researcher employed to achieve these objectives and positions the study within the broader assessment of factors responsible for enhancing the effectiveness of weight loss programs.
Research Objects
From January 2021 to May 2022, obese and overweight patients in the Medical Weight Loss Clinic, Department of Endocrinology, Xuanwu Hospital, Capital Medical University, participated in this study. These groups of participants were classified according to the patients’ wishes after they were briefed about the three dietary plans for the intervention program. Patients who had lost weight through drugs and surgery were excluded from the investigation, leaving only 122 cases in the energy-restricted balanced diet group, 92 cases in the high-protein diet group, and 262 cases in the light-fasting diet group.
Study Design and Participants
Doctors, nutritionists, and patients established a WeChat group of three to participate in the study. They regularly guided patients on how to implement prescribed dietary patterns. A follow-up visit was scheduled once a month, and another meeting was planned for 3-6 months after operations began.
Changes in BMI, weight, and body composition were measured at the first and at each subsequent visit thereafter. Relative to this plan, the researcher compared changes in waist and hip circumferences among the three intervention groups described in this paper. According to the diagnostic criteria, overweight patients have a BMI between 24 and 28, while obesity is defined as a BMI greater than 28. These health indicators were instrumental in determining the effectiveness of interventions.
Data Collection
The researcher used the Body Composition Meter to gather information about the study. This data was developed using the in-Body 270 machine from in-Body Company. This strategy was tailored to meet the study’s objectives, which were to elucidate the effects of high-protein diets on fat and muscle loss in obese patients. This plan was consistent with the views of scholars who recommend that the process of determining the best data collection technique to use should align with the study’s goal. Consistent with this purpose, information gathering occurred in the Chinese language; however, the collected data was later translated into English for consistency in reporting and ease of data analysis.
Statistical Analysis
The researcher used the Microsoft Excel method to analyze data. Its selection was informed by its inherent capabilities to visualize data through graphical analysis. From this background, this technique enabled the researcher to simplify complex data into information silos, facilitating ease of data analysis. It ensured that the measurement data process conformed to established statistical techniques for data analysis. They were represented by the mean value of the variables, plus or minus their variance. The comparison of data between two groups of users was completed using the independent sample t-test, and the assessment of multiple groups was completed using the single-factor ANOVA analysis of variance technique. The reference value range was between 2.5 and 97.5, implying that where P<0.05, the difference is statistically significant.
Results
Basic Information of Research Objects
As highlighted in this study, the present inquiry is divided into three groups of experiments, each representing a distinct dietary plan. For example, 122 cases were associated with the energy-restricted diet pattern group. It involved 95 female cases and 27 male cases, with an age range of 20 to 64 years. In the high-protein diet category, 92 cases were examined, comprising 88 female and four male cases. The age range of the respondents was between 22 and 44 years. In the light fasting dietary pattern group, 262 cases were recorded, comprising 211 female and 51 male cases. The respondents’ ages ranged between 21 and 52 years.
Comparison of Changes in Body Composition Before and After Intervention
Weight differences, skeletal muscle mass, BMI, and body fat indices were the main variables used to monitor changes in weight loss outcomes in this study. These variables were based on the three dietary interventions highlighted above: high-protein, calorie-restricted, and light fasting diet groups. The information associated with the interventions includes BMI, SMI, muscle loss, and fat loss. As stated in the first chapter of this document, SMI and muscle loss were the main variables investigated in the study. The impact of high-protein diet treatments on efficacy is explained in subsequent sections of the study.
SMI
The mediating role of SMI factors in obesity management is crucial to this study, as it highlights changes in muscle mass resulting from dietary interventions. The present study examined the effect of high-protein diets on SMI to estimate the impact of such dietary provisions on non-fat mass. Figure 1.1 below shows weekly changes in SMI throughout the intervention period.
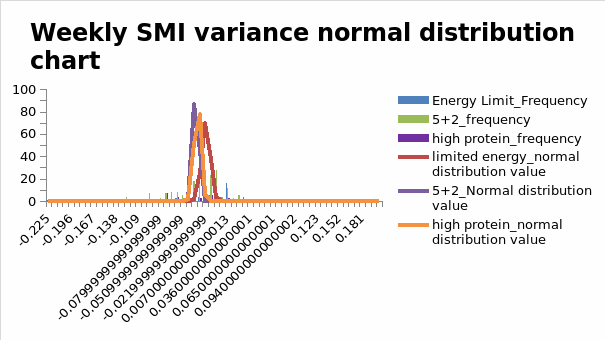
Figure 1.1 above shows that the normal distribution of associated data was equally spiked during the intervention period. This statement implies that SMI marginally decreased below the expected levels
The findings derived from this investigation are consistent with those of others that have affirmed the impact of weight loss interventions on changes in SMI. They suggest that the effects of SMI vary with the core purpose of the intervention. Therefore, interventions with inconsistent results are likely to maintain their efficacy if they are coupled with those aimed at increasing participation rates.
Scholars who have investigated the same effect on SMI outcomes support these statements. However, they emphasize the importance of evaluating the nutritional compounds of the diets to understand their overall effectiveness. For example, the diets of obese patients commonly have low fiber levels and are characterized by low intensities of fruit consumption. Within the same lens of analysis, some patients have C-reactive protein in their genetic makeup, which may either worsen or improve their metabolic reactions to high-protein diets. Such metabolic agents may lead to variations in skeletal muscle differences during analysis.
The findings highlighted above indicate that the three different dietary programs led to significant changes in SMI outcomes. They are consistent with research investigations, which have shown that a high-protein diet is associated with the preservation of lean body mass. However, the effects of such a diet on non-fat mass are inconsistent across studies, with some indicating a decrease in this measure, while others suggest an increase. Nevertheless, variations in the findings emerged from nuances in intervention implementation.
Weight Differences
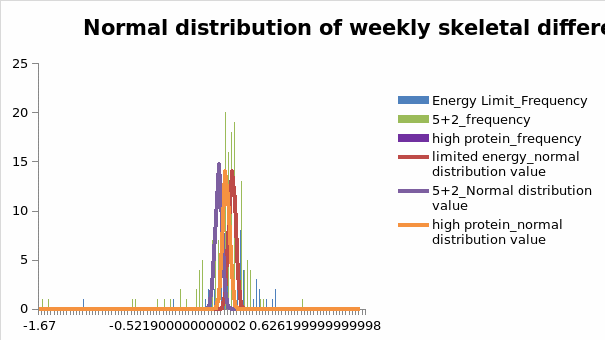
It was essential to record differences in weight among the informants because it was a reliable indicator of changes in fat levels. Figure 1.2 below shows the findings for the normal distribution of weekly weight differences.
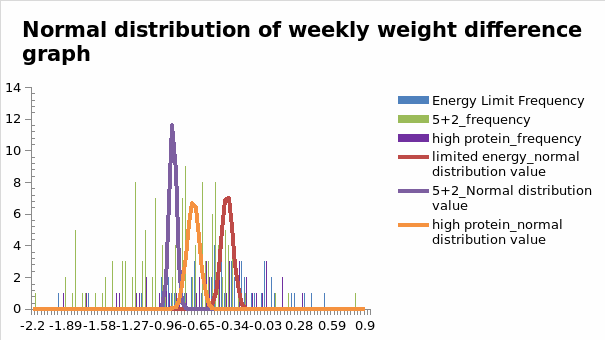
Consistent with the findings on the impact of SMI on weight loss programs highlighted above, Figure 1.2 shows significant changes in weekly weight differences for informants who participated in the high-protein intervention program. This finding means that the program’s effectiveness was heightened during the intervention plan. Figure 1.2 above equally shows that weight differences were more evenly spread across the intervention period compared to other biological indicators sampled in the study. These findings suggest that weight loss was achievable after prolonged exposure to the program.
The above findings reveal that fat levels decreased throughout the intervention period. However, weekly weight distribution levels among high-protein diets were the highest. This variation in outcome, compared to other dietary interventions included in the study, underscores the importance of understanding factors that may contribute to weight loss in diet-based interventions.
For example, weight changes were partly moderated by exposure to medical procedures, such as those who have undergone treatment for maxillofacial fractures. These medical procedures are associated with significant long-term weight reductions. Consequently, a high-protein diet would help to rebalance weight. These findings were mirrored in the present investigation, as highlighted above.
Body Fat
Weight gains and losses are often accompanied by changes in fat levels, including both visceral and subcutaneous fat. Therefore, the use of this biological indicator to assess the impact of a high-protein diet on weight loss treatment was critical in isolating the effects of muscle loss during the intervention program. In relation to this assertion, restricting calorie intake and increasing physical activity levels are two non-surgical ways to reduce weight. Patients who participated in such weight loss plans often strived to improve their health outcomes by maintaining a healthy Body Mass Index (BMI). As alluded to in this study, this biological indicator assesses their weight relative to their height and age.
Subject to the aforementioned effects of dietary interventions on weight loss, some weight loss strategies adopted by healthcare practitioners to manage obesity involve modifying protein-based diets. The same strategy has been used to assess the effectiveness of protein-based diets in weight loss programs. For example, researchers who have used diet interventions aimed at promoting weight loss through variations in protein levels have used nitrogen balance as a key determinant of clinical effectiveness. These developments highlight the potential effect of dietary changes on the outcomes of weight management programs. Figure 1.3 below illustrates significant differences in the standard distribution chart of weekly body fat percentage, as indicated by the findings.
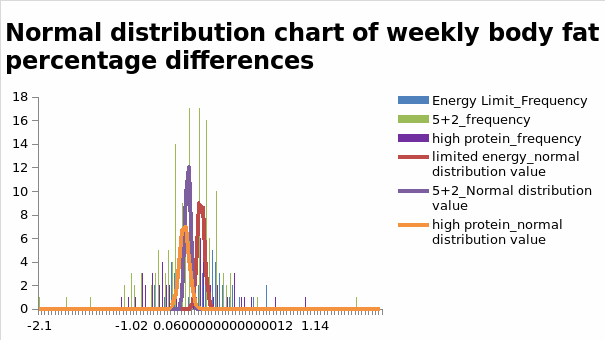
The findings highlighted above indicate that changes in body fat levels between -0.1 and -0.5 materialized in the experiment. This range indicates that the high-protein diet resulted in significant fluctuations in fat levels. Given the relationship between body fat levels and BMI, the present analysis further examined changes in BMI. Stated differently, variations in fat mass were further compared with differences in BMI levels. The results are highlighted in Figure 1.4 below.
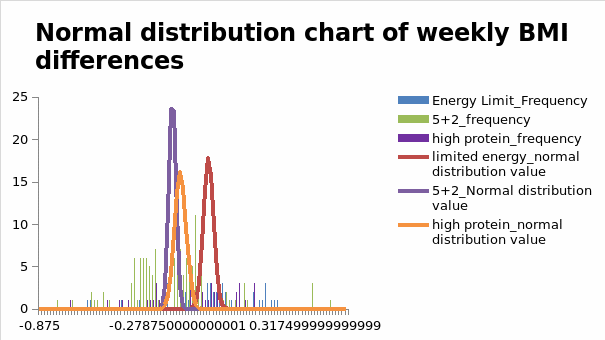
The frequency of high-protein diets was established in the high-protein diet category of the analysis. Similarly, the normal distribution of high protein data was located within the same BMI range. The interventions employed in the study resulted in a significant reduction in body weight of 0.73 kg/m². This figure translates to an equivalent weight loss of about 2.5 kg. The reduction in body mass was associated with a decrease in the waist circumference of the patients sampled. However, the overall change is significantly lower than those reported in other studies, which have registered BMI changes of up to 30kg/m2.
Broadly, the findings highlighted above showed that the high-protein diet intervention did not alter the BMI requirements for the patients, as they were all below 25kg/m². The ideal BMI for adults is within the 18.5 kg/m2 to 24.9kg/m2 range. Conversely, a BMI of more than 25kg/m² indicates that one is overweight, and a BMI of 30kg/m² or higher implies that a person is clinically obese. Overall, these findings suggest that high-protein diets resulted in significant gains in BMI, which fell within the healthy range. The assumption to be made here is that the program led to a reduction in fat and muscle mass.
Skeletal Muscle Differences
The relationship between skeletal muscle and obesity stems from concerns about the potential loss of muscle mass during weight loss management programs. Scholars suggest that people who suffer from losses in skeletal muscle are prone to several adverse health outcomes, including low life expectancy and high rates of acute or chronic illnesses. Their views draw attention to the effects of obesity on skeletal muscle by suggesting that weight gain leads to a deterioration of the same health indicator.
Similarly, researchers suggest that obesity may lead to lower bone densities, and the effects are further worsened with aging. Most studies that have explored this area of research have taken a keen interest in the impact of gender and age-related factors on explaining the effect of dietary interventions on weight reduction programs. For example, scholars suggest that women tend to lose skeletal muscle density faster than men do. This outcome persists across various treatment modalities.
The investigation recorded differences in skeletal muscle to ascertain the impact of the high-protein diet on non-fat mass. The findings are presented in Figure 1.5 below, which shows changes in skeletal muscle during the intervention period.
As highlighted above, the high-protein intake diet led to a marginal reduction in skeletal muscle mass during the intervention period. These findings indicate that reductions in non-fat mass were associated with the intervention program. The present outcome is derived from the low changes in muscle indicators along the Y-axis of the graph compared to the BMI chart.
Based on the above findings, scholars suggest that patients who suffer from losses in skeletal muscle due to weight loss treatments have a high probability of developing Sarcopenic obesity. As its name suggests, this illness is associated with the loss of skeletal muscle mass during treatment. These claims stem from research studies that indicate some types of obesity management programs may cause skeletal muscle contraction. This outcome is commonly associated with the loss of lean mass, resulting from a mismatch between fat and skeletal muscle volumes. In this assessment, the risk of suffering losses in skeletal muscle increases with age.
Drawing from the link between lean muscle loss and dietary changes, some observers believe that the occurrence of Sarcopenic obesity stems from the double occurrence of obesity and Sarcopenia among patients. This disparity could account for the high number of physical health injuries reported among victims because a poor state of skeletal muscle is associated with disability and physical unfitness.
Thus, it could be argued that SMI is responsible for the rising mortality gap between obese patients and the general population. Alternatively, this assumption means that Sarcopenic obesity may become a significant health issue in the future. These insights suggest that the effects of dietary changes on weight loss programs should be assessed based on their impact on both non-fat and fat mass loss.
Overall, the findings depicted in this chapter reveal that reductions in SMI and muscle mass occurred during the intervention period. However, compared to fat mass, these changes were relatively insignificant. This finding suggests that high-protein diets result in changes in both fat and non-fat mass, with a relatively lower loss of muscle mass. The reasons for this outcome, including a comparative analysis of these findings with the existing body of literature and an examination of the merits and demerits of the processes used to generate the findings, will be explored in subsequent chapters.
Discussion
Dietary Treatments and Obesity
The increased use of interventions for weight loss management stems from research studies that have linked changes in food portions to positive health outcomes, such as a reduction in high blood pressure and a lower risk of developing diabetes. However, most of the attention directed at improving weight loss outcomes has been on restricting dietary intake as the sole method of realizing weight changes. Consequently, nutritionists have proposed different dietary interventions to treat obesity. Broadly, their proposals have described calorie-restricted and high-protein diets as the two main weight loss strategies. In both dietary interventions, the primary health goal is to limit fat loss rather than muscle loss.
In relation to the developments above, some researchers have noted that the omission of a nutritional profile analysis in weight loss plans has resulted in nutritional insufficiency in current weight loss programs. For example, a US-based study that analyzed 20 essential micronutritional properties in weight loss plans revealed significantly low levels of calcium, zinc, and selenium in their intervention program. Likewise, the study revealed that micronutritional deficits were commonly associated with most weight-loss management programs in the US. Thus, it was recommended that nutritional analysis be pivotal in understanding the efficacy of existing dietary plans.
The importance of dietary review in assessing the impact of weight loss interventions has been mentioned in the context of the current probe. Notably, the researchers evaluated the impact of peanut consumption on weight loss outcomes. The intervention was conducted over six months and involved adults with low-risk exposure to Type 2 diabetes.
The findings demonstrated that consuming peanuts before meals resulted in a significant weight reduction. Thus, the dietary intervention was proposed as a viable method of managing obesity. Broadly, these findings highlight the importance of understanding the impact of nutritional properties on weight loss management. The same approach was applicable in the present study to understand the impact of high-protein diets on fat and non-fat weight loss.
The Role of High-Protein Diets in Effective Weight Management
Naturally, the efficacy of high-protein diets in promoting weight loss is predicated on making changes to one’s dietary intake. The desired human average protein requirement is between 0.75 g/kg and 0.8 g/kg, thereby providing a basis for evaluating changes to weight levels emerging in this study. These standards of protein intake help detect discrepancies between average and abnormal protein levels.
For example, the habitual daily intake of food among obese patients is estimated to surpass the standard protein level administered in the current intervention. At the same time, certain population groups may require higher levels of protein consumption due to age-related factors, such as the elderly or individuals with pre-existing health conditions. Relative to this assertion, studies have shown that older people suffer a significant risk of skeletal and muscle tissue loss due to age-related factors. Therefore, they may require a higher protein intake compared to the general population.
Patients with high-energy requirements also have a higher protein need compared to the rest of the population. For example, endurance athletes require a high intake of protein in their diets due to their high physical energy requirements. This observation has been supported by research investigations, which have examined the impact of body composition goals and weight loss requirements among rugby players in New Zealand. In the study, it was reported that differences in body composition are linked to changes in fat and weight levels. These findings suggest that the interventions adopted in the study indicate that the efficacy of dietary interventions may have differed in terms of achieving body composition goals.
As highlighted above, the expectation that obesity management could be improved through variations in protein intake has gained support in the present scholarly literature. However, most research attention has been skewed towards determining how protein intake can suppress hunger and enhance satiety, rather than reducing weight. Consequently, many studies have not examined the effects of protein-specific diets on weight loss management programs.
Instead, attention has been paid to promoting the intake of protein-rich foods above the recommended daily intake levels to promote muscle and minimize fat gain. In relation to this assertion, the importance of assessing the quality of protein intake in dietary management as a basis for understanding its effectiveness in weight loss management is emphasized. However, this line of research has not gained traction in present scholarly research.
The type of food administered in the dietary interventions may have similarly influenced the findings. For example, dairy products and eggs have been used as protein-rich foods in weight loss therapies. Indeed, a study aimed at ascertaining the impact of high-protein diets on the weight reduction efforts of professionals found that significant reductions in weight levels occurred in representative groups over 16 weeks. Participants who consumed the high-protein and high-dairy meals reported significantly lower levels of fat loss over the same period. These findings suggest that the type of protein diet given to patients affects their weight loss outcomes.
The insignificant reduction in muscle mass observed in this investigation is consistent with existing research, which has also reached the same conclusion, albeit using calorie-restricted diets. They suggest that if non-obese patients were to experience the same therapy, they are likely to lose about 35% of their overall body mass. Comparatively, obese people have relatively higher levels of muscle and fat mass than the average person. Therefore, they are likely to experience a lower rate of muscle mass loss compared to the average patient.
Concisely, research reports indicate that these patients may lose between 20% and 30% of their fat-free mass through dietary interventions. This argument lends credence to the idea that food properties play a crucial role in determining the extent of changes in fat and muscle loss. Therefore, portions of the protein diet associated with the intervention programs could have had an impact on the findings. This outcome was determined after it was established that providing patients with adequate protein and low-dairy foods resulted in a loss of lean mass.
The findings mentioned above imply that weight loss reductions are realizable only when a whole-food approach is adopted as a dietary intervention. In other words, a high intake of protein and dairy is necessary to maintain lean mass, body strength, and fitness. Despite the informative nature of the findings mentioned above, it is essential to interpret the findings cautiously because they were specifically tailored to reflect the unique health settings of Chinese patients and hospitals. Therefore, they do not provide a holistic picture of the effects of high-protein diets on muscle loss for all patients.
In this investigation, it is essential to account for the effects of aging on the findings, as this demographic variable is responsible for variations in visceral fat levels, which may impact computations on muscle and fat mass loss. At the same time, aging is associated with the progressive loss of muscle mass, which should similarly be accounted for in the analysis. In this regard, the importance of evaluating the impact of dietary interventions on muscle loss patterns cannot be underestimated because it is a risk factor when determining mortality levels among obese patients. From these insights, it can be deduced that a high-protein diet preserves lean body and muscle mass due to the inherent properties of protein foods, which are to build muscle tissue, as opposed to a calorie-based diet, which seeks to minimize fat levels.
Based on the above insights, it can be assumed that high-protein diets are more effective in targeting fat as opposed to muscle loss. The same effects are also applicable to muscle strength and the metabolic rates of the patients, as it is likely that the protein-based diet helped preserve lean body mass. Relative to these findings, researchers have associated poor muscle quality with obese patients. This characterization of the patient’s health data indicates that the same population group may require higher protein intake to compensate for their loss.
Although gender was not a significant variable in analyzing the results of this study, evidence suggests that men are more likely to experience a higher loss of muscle mass compared to their female counterparts. This muscle loss is most substantial immediately after the introduction of weight loss treatments. Likewise, it is speculated that men’s lean body accounts for the difference in health outcomes between the female and male genders. Therefore, gender-related factors play a role in understanding the influence of health-based interventions on fat and muscle loss. However, given that the number of male and female participants in the program was balanced, it can be assumed that the effects of gender were insignificant.
Relationship Between Muscle Loss and Obesity
The losses in skeletal muscle witnessed in the present study are closely linked with findings that have identified vulnerable groups as beneficiaries of specialized care during health emergencies. For example, elderly patients suffering from obesity formed a special interest group requiring specialized care when designing weight loss management programs. Thus, age-related barriers should be eliminated to achieve equity in health outcomes.
The findings of this investigation affirmed the view that the risk of skeletal muscle loss is high among elderly population groups compared to others. Therefore, there is a need to provide specialized care to at-risk groups. Scholars who have investigated the same issue have found that losses in skeletal muscle mass negatively affected the relationship between in-person weight programs and obesity management interventions.
Stated differently, it was established that losses in bone density negatively influenced quality of life. The study aimed to investigate the effectiveness of in-person weight management programs on treatment efficacy. The findings from this study emphasized the importance of recognizing the impact of weight reduction programs on skeletal muscle loss in obese patients, aiming to improve their overall quality of life.
Impact of SMI on Obesity Treatment
The findings of this study indicate that high-protein diets have a significant impact on skeletal muscle mass. These results are consistent with those of other studies, which have shown a similar effect on other dietary interventions, such as high-calorie diets. Several reasons could explain changes to SMI in weight loss programs. However, as highlighted in the literature review section of this study, some researchers attribute such differences to age and gender-related factors, with some reports indicating that general age-related factors are likely to cause losses in skeletal muscle density regardless of the treatments offered. Thus, it is essential to understand the role that the respondents’ demographic profile played in generating the findings outlined in this document.
Given that the respondents were relatively young, with an average age of below 50 years, the changes in SMI observed in the study were primarily attributable to the dietary interventions, rather than the respondents’ ages. These results have been nurtured from investigations that have tested the efficacy of dietary strategies for weight reduction. Consistent with this assertion, the results of the current investigation demonstrate that high-protein diets have an influence on SMI levels.
Broadly, the findings mentioned above are consistent with research investigations, which affirm a positive relationship between a high-protein diet and losses in non-fat mass. Similarly, they counter the purpose of initiating the weight loss program in the first place because losses in non-fat mass are undesirable and could lead to ill health and disability. Thus, it is unclear why there were significant disparities in SMI change across the sample population group. However, it can be assumed that person-specific factors contributed to this discrepancy in outcomes. This observation highlights the importance of precision medicine in managing obesity, as it considers individual factors when designing interventions.
At the same time, endurance and resistance-style interventions could play a pivotal role in minimizing the adverse effects of muscle loss in high-protein dietary plans. These interventions could be implemented by including innovative digital health tools in obesity management. Such an approach could increase engagement between obese patients and their healthcare service providers.
Desired goals should equally be directed at improving the health outcomes of patients suffering from weight-related health complications. In particular, healthcare practitioners and policy experts should consider introducing targeted lifestyle interventions that appeal to the needs of the patients. This contextualized approach to healthcare development can be adopted at both policy and administrative levels, as it may impact resource allocation and overall program effectiveness.
Targeted lifestyle changes may be adopted as a preventive action to minimize exposure to risk factors associated with obesity. The process may involve modifying lifestyle behaviors in patients with the condition. However, patients may be reluctant to adopt such interventions because the general population has been unable to achieve positive health outcomes through lifestyle interventions in the first place. Therefore, one may question how patients who are obese are expected to register improved results by adopting new lifestyle activities. Nonetheless, developing targeted lifestyle interventions for patients suffering from various health issues would help build confidence in the ability of public health interventions to address social and environmental factors that influence patients’ lifestyles.
In this analysis context, collaboration across different sectors and interest groups may be pursued to make the lived environment appropriate for residents to maintain an active lifestyle. This recommendation is based on the work of researchers who have recognized the importance of the relationship between the lived environment and the incidence of obesity. The author also reminds us that obesity is a lifestyle disease, and only a holistic approach to addressing its key drivers is likely to yield the most promise in terms of positive health outcomes.
Study Limitations
The data indicate that the proportion of overweight individuals in the energy-restricted balanced diet group is higher than that of obese individuals in the high-protein diet category, which may impact the statistical significance of the results. Additionally, the overall number of patients recruited in the study is small.
However, this limitation is not unique to the study because the use of high-protein diets to determine weight loss outcomes has been undermined by the use of small sample sizes in many scholarly works. For example, the use of high-protein diets for weight loss management was linked with low levels of lean tissue growth among 12 respondents in an 8-week weight loss reduction program. Despite its robust findings, the small sample population used in the study undermined the ability to generalize the data. This limitation applies to the present investigation, thereby contributing to the view that the sample population limited the ability to generalize results.
An additional limitation of the study was related to the selection of groups. In other words, it was assumed that respondents fully understood the diet plans. Therefore, they chose one that best suited their quest to achieve desired health outcomes. This strategy of participant recruitment means that the investigation was not a prospective study or random grouping, hence limiting the ability to generalize its findings. Overall, the small sample size and research design used in the present investigation affected the ability to generalize the findings.
Conclusion
Dietary interventions are a source of knowledge for understanding metabolic processes associated with weight loss programs. The present study evaluated the efficacy of high-protein, low-carbohydrate, low-fat (HPLCF) diets and CRD diets on weight loss, relative to their impact on changes in muscle and fat mass. The results showed that all three dietary patterns were effective in managing body weight. However, the light fasting group achieved the most significant reductions in body weight and BMI, followed by the high-protein group, both demonstrating more pronounced effects than the energy-restricted balanced diet group.
The reduction in fat mass in the three groups was significantly lower than that of muscle mass; however, fat mass decreased significantly in the high-protein diet group compared to the other two groups. The energy balance diet with reduced muscle mass was the least affected, while the light fasting diet was the most affected; however, no significant difference was found among the three intervention groups studied. Based on these results, a high-protein diet appears effective in reducing body fat mass while preserving muscle mass.
The unique weight loss outcomes associated with the protein-based diet investigated in the current study underscore the importance of understanding the dietary properties of foods. The same logic applies to understanding the impact of other dietary interventions, such as CRD, on changes in body weight. Relative to this assertion, the findings of the present investigation suggest that protein-based diets exhibit lean preservation properties, as they minimize muscle loss compared to fat loss. Therefore, the relatively low changes in muscle mass compared to fat mass could be attributed to this fact.
Based on the insights highlighted above, high-protein diets portend significant benefits to patients suffering from obesity. They promote their ability to preserve muscle mass and enhance fat loss during intervention programs. However, focus should be given to determining the optimal amount of nutritional content that should be included in protein-based diets to achieve positive health outcomes. Such suggestions underscore the importance of delivering personalized healthcare solutions tailored to the diverse needs of patients. Nonetheless, from a practitioner’s perspective, the insights presented in this analysis create room for a more detailed discussion on the effects of high-protein diets on muscle and fat mass.
References
- Hachem F, Vanham D, Moreno LA. Territorial and sustainable healthy diets. Food and Nutrition Bulletin. 2020; 41(2):87-103.
- American Association of Clinical Endocrinology. (2022) Clinical Practice Guidelines. Web.
- Holt RG. The management of obesity in people with severe mental illness: an unresolved conundrum. Psychotherapy and Psychosomatics. 2019; 88(2): 327–332.
- Whittaker J. High-protein diets and testosterone. Nutrition and Health. 2022; 7(2): 231-244.
- Bansal P, Bhandari U, Sharma K, Arya P. Embelin modulates metabolic endotoxemia and associated obesity in high fat diet fed C57BL/6 mice. Human & Experimental Toxicology. 2021;40(1):60-70.
- Stokes P. Research methods. London: Palgrave Macmillan; 2017; 1-28.
- Winston W. Microsoft Excel 2019 data analysis and business modeling. New York, NY: Microsoft Press, 2019; 78-134.
- McFedries P. Excel data analysis for dummies. London: John Wiley & Sons; 2018; 109-295.
- Wilson K. Using excel 2019: the step-by-step guide to using Microsoft excel 2019. London: Elluminet Press; 2020; 14-138.
- MacFarland TW, Yates JM. Using R for biostatistics. New York, NY: Springer Nature; 2021; 1-19.
- Spilsbury L, Spilsbury R. The skeleton and muscles. London: Raintree; 2018; 22-30.
- Burniston JG, Chen Y. Omics approaches to understanding muscle biology: methods in physiology. New York, NY: Springer Nature; 2019; 79-109.
- Li X, Shi W, Xiong Q, et al. Leptin improves intestinal flora dysfunction in mice with high-fat diet-induced obesity. Journal of International Medical Research. 2020;48(6): 445-454.
- Pocovi-Gerardino G, Correa-Rodríguez M, Rubio J-LC, Fernández RR, Ortego-Centeno N, Rueda-Medina B. Diet quality and high-sensitivity c-reactive protein in patients with systemic lupus erythematosus. Biological Research for Nursing. 2019; 21(1):107-113.
- Wright CS, Li J, Campbell WW. Effects of dietary protein quantity on bone quantity following weight loss: a systematic review and meta-analysis. Advances in Nutrition. 2019; 10(6): 1089-1107.
- Lone PA, Khaliq M, Sharma M, Malik OA, Lone BA. Weight changes (in kg) in mandible fracture patients after IMF: a prospective study. The Traumaxilla. 2019;1(1):35-37.
- Kasprzak K, Wojtunik-Kulesza K, Oniszczuk T, Kuboń M, Oniszczuk A. Secondary metabolites, dietary fiber and conjugated fatty acids as functional food ingredients against overweight and obesity. Natural Product Communications. 2018;13(8): 112-119.
- Siino V, Jensen P, James P, Vasto S, Amato A, Mulè F, Accardi G, Larsen, MR. Obesogenic diets cause alterations on proteins and theirs post-translational modifications in mouse brains. Nutrition and Metabolic Insights. 2021; 14(7), 1-10.
- Javed A, Kostas N. Advances in psychiatry. New York, NY: Springer; 2018; 1-19.
- Fang J, Xu B. Blood urea nitrogen to serum albumin ratio independently predicts mortality in critically ill patients with acute pulmonary embolism’, Clinical and Applied Thrombosis/Hemostasis. 2021; 27(8): 112-119.
- Edda C, Nai CY, Mittendorfer B. Preserving healthy muscle during weight loss. Advances in Nutrition. 2017; 8(3): 511–519. Web.
- Xiao J. Muscle atrophy. London: Springer; 2018; 423-444.
- Cruz-Jentoft A, Morley, J. Sarcopenia. London: John Wiley & Sons; 2021; 199-227.
- Ogura Y, Sato S, Gallot YS, Arthur ST. Emerging mechanisms for skeletal muscle mass regulation. London: Frontiers Media SA; 2021; 73-85.
- Sivanand A, Gulliver WP, Josan CK, Alhusayen R, Fleming PJ. Weight loss and dietary interventions for Hidradenitis Suppurativa: a systematic review. Journal of Cutaneous Medicine and Surgery. 2020; 24(1):64-72.
- Oliver C. Muscles do matter: aging well – aging strong. London: eBookIt.com; 2021; 17-87.
- Maggi L, Matthews E, Desaphy, J. New insights in skeletal muscle channelopathies – a rapidly expanding field. London: Frontiers Media SA; 2021; 5-20.
- Afzal M, Siddiqi N, Ahmad B, et al. Prevalence of overweight and obesity in people with severe mental illness: systematic review and meta-analysis. Frontiers in Endocrinology 2021; 12(25): 1-10.
- Goldstein SP, Hoover A, Evans EW, Thomas JG. Combining ecological momentary assessment, wrist-based eating detection, and dietary assessment to characterize dietary lapse: A multi-method study protocol. Digital Health. 2021; 7(2): 334-342.
- Engel M, Kern H, Brenna JT, Mitmesser S. Micronutrient gaps in three commercial weight-loss diet plans. Nutrients. 2018; 10(1):108.
- Semlitsch T, Jeitler K, Berghold A, Horvath K, Posch N, Poggenburg S, Siebenhofer A. Long-term effects of weight-reducing diets in people with hypertension. The Cochrane Database of Systematic Reviews. 2016; 3(3): 261-274.
- ElhamKia M, Setayesh L, Yarizadeh H, et al. The interaction between dietary total antioxidant capacity and mc4r gene and homa-ir in metabolically healthy and unhealthy overweight and obese women. Nutrition and Metabolic Insights. 2022; 15(3): 1-11.
- Petersen KS, Murphy J, Whitbread J, Clifton PM, Keogh JB. The effect of a peanut-enriched weight loss diet compared to a low-fat weight loss diet on body weight, blood pressure, and glycemic control: a randomized controlled trial. Nutrients, 2022; 14(14); 298-306.
- May M, Schindler C, Engeli, S. Modern pharmacological treatment of obese patients. Therapeutic Advances in Endocrinology and Metabolism. 2020; 11(3): 1-12.
- Foley PJT, Gunson JTS, Baumann SL. Two stories about diet and diabetes in Europe. Nursing Science Quarterly. 2020; 33(1): 85-90.
- Morgan PT, Smeuninx B, Breen L. Exploring the impact of obesity on skeletal muscle function in older age. Frontiers in Nutrition. 2020; 7(5): 69-89.
- Black KE, Hindle C, McLay-Cooke R, Brown RC, Gibson C, Baker DF, Smith B. Dietary intakes differ by body composition goals: an observational study of professional rugby union players in New Zealand. American Journal of Men’s Health. 2019; 13(6): 223-234.
- McCarthy D, Berg A. Weight loss strategies and the risk of skeletal muscle mass loss. Nutrients. 2021; 13(7): 2473-2477.
- Verreijen AM, Engberink MF, Memelink RG, van der Plas SE, Visser M, Weijs PJ. Effect of a high protein diet and/or resistance exercise on the preservation of fat free mass during weight loss in overweight and obese older adults: a randomized controlled trial. Nutrition Journal. 2017; 16(1): 1-10. Web.
- Kossoff E, Cervenka M. Ketogenic dietary therapy controversies for its second century. Epilepsy Currents. 2020; 20(3):125-129.
- Kim EJ, Sohn TS, Choi HH, et al. High levels of Akkermansia muciniphilia growth associated with spring water ingestion prevents obesity and hyperglycemia in a high-fat diet-induced mouse model. Natural Product Communications. 2022; 17(7): 232-246.
- Joseph G, Arviv-Eliashiv R, Tesler R. A comparison of diet versus diet plus exercise programs for health improvement in middle-aged overweight women. Women’s Health. 2020; 16(2): 119-126.
- Juarez E, Tufiño C, Querejeta E, Bracho-Valdes I, Bobadilla-Lugo RA. Evidence of changes in alpha-1/AT1 receptor function generated by diet-induced obesity. Diabetes and Vascular Disease Research. 2017; 14(6):485-493.
- Mier N, Smith ML, Wang X, Towne SD, Carrillo G, Garza N, Ory, MG. Factors associated with diet and exercise among overweight and obese older Hispanics with diabetes. SAGE Open. 2017; 77(2): 1-12. Web.
- Simonson, M., Boirie, Y. and Guillet C. (2020) ‘Protein, amino acids and obesity treatment,’ Reviews in Endocrine and Metabolic Disorders, 21(3), pp. 341-353.
- Sakuma K. Muscle cell and tissue: current status of research field. Books on Demand; 2018; 7-63.
- Nakamura T. The impact of diet type on weight loss in patients with Alzheimer’s disease. American Journal of Alzheimer’s Disease and Other Dementias. 2021; 3(6): 1-12.
- Bomberg EM, Ryder JR, Brundage RC, et al. Precision medicine in adult and pediatric obesity: a clinical perspective. Therapeutic Advances in Endocrinology and Metabolism. 2019; 10(2): 231-243.
- Price JC, Santos HO, Bueno AA. The effectiveness of automated digital health solutions at successfully managing obesity and obesity-associated disorders: a PICO-structured investigation. Digital Health. 2022; 8(2): 1-10.
- Harrison MR. Effects of public–private partnership on diet-related obesity risk factors among school-aged children: a systematic literature review. Nutrition and Health. 2022; 7(2): 120-129.
- Patten L, Newhart M. Understanding research methods: an overview of the essentials. London: Taylor & Francis; 2017; 1-17.