Casein and whey are two forms of proteins present in milk that determine the design of food formulas and the processing of dairy products to meet the diverse needs of consumers. Depending on their sources, casein and whey proteins vary in their concentrations in cow, human, and goat milk. The research question of the experiment is what is the concentration of lysine in whole and whey portions of goat milk?
Results
The calculated concentrations of BSA standards have an increasing trend from 0 µM to 80 µM in each of the 2500 reaction contents in tubes 1-5 and samples used in the measurement of absorbance. The standard line (Figure 1) depicts a linear relationship between the absorbance of primary amine groups in proteins at 570 nm and the concentration of BSA standards.
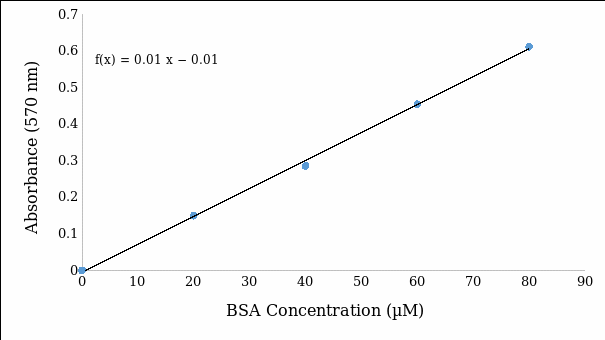
As the linear equation derived from the standard line, absorbance = 0.0076 (Concentration) – 0.0052. In this view, this linear equation suggests that a rise in the concentration of BSA standard by a microliter causes the absorbance to increase by 0.0076 units. Based on the formula [concentration = (absorbance/ 0.0076) – 0.0052], concentrations of samples revealed that a fifty-fold dilution of whey has 62.11 µM, while a hundred-fold dilution of milk has 66.19 µM (Table 1).
Table 1. Absorbance values of whey and milk samples with their respective concentrations
Results (Table 2) indicate that whey and milk have concentrations of 3105.523 µM and 6618.941 µM of lysine. Moreover, the findings show that lysine in whey constitutes 46.92% of the proteins in goat’s milk.
Table 2. Concentrations of lysine in various dilutions and original samples of whey and milk
Supplementary Materials
Calculations of Amine Concentrations in Bovine Serum Albumin (BSA)
Concentrations of BSA dilutions (C2) were determined using C2 = C1V1/V2 formula
C1 = 200 µM amines in BSA standard
V2 = 2500 µL
BSA concentrations, V1 = 0 µL, 250 µL, 500 µL, 750 µL, and 1000 µL
For tube 1 (blank), C2 = (0 µL × 200 µM)/2500 µL = 0 µM
For tube 2, C2 = (250 µL × 200 µM)/2500 µL = 20 µM
For tube 3, C2 = (500 µL × 200 µM)/2500 µL = 40 µM
For tube 4, C2 = (750 µL × 200 µM)/2500 µL = 60 µM
For tube 5, C2 = (1000 µL × 200 µM)/2500 µL = 80 µM
Calculations of Concentrations Proteins in Whey and Milk
Based on the linear trend line formula from excel, the following is the standard line equation. Since y = mx + b, the standard line equation, which represents absorbance = slope (concentration) + y-intercept, is y = 0.0076 – 0.0052. By substituting values into the equation, absorbance = 0.0076(Concentration) – 0.0052.
Therefore, concentration = (absorbance/0.0076) + 0.0052
For whey sample, concentration = (0.472 /0.0076) + 0.0052 = 62.1053 + 0.0052 = 62.1105
For milk sample, concentration = (0.503/0.0076) + 0.0052 = 66.1842 + 0.0052 = 66.1894
The results of the analysis were recorded in the datasheet below to shows the concentration of amines in BSA standards, whey, and milk, as well as their respective absorbance values.
Data Sheet
Calculations of Amine Concentrations
The concentration of Amines in Whey
- The concentration of amines in the tube sample of whey
Concentration = (0.472/0.0076) + 0.0052 = 62.1053 µM
- The concentration of amines in the dilute sample of whey
The dilution ratio of dilute sample to whey tube sample is 1:5
Concentration = 62.1053 × 5 = 310.5523 µM
- The concentration of amines in the original sample of whey
The dilution ratio of the original sample to dilute sample is 1:10
Concentration = 310.5523 × 10 = 3105.523 µM
The concentration of Amines in Milk
- The concentration of amines in the tube sample of milk
Concentration = (0.503/0.0076) + 0.0052 = 66.18941 µM
- The concentration of amines in the dilute sample of milk
The dilution ratio of dilute sample to milk tube sample is 1:10
Concentration = 66.1894 × 10 = 661.8941 µM
- The concentration of amines in the original sample of milk
The dilution ratio of the original sample to dilute sample is 1:10
Concentration = 661.894 × 10 = 6618.941 µM
Percent of Lysine in Whey
Percent of lysine in whey = [original sample of whey]/[original sample of milk] × 100% = 3105.523 µM/6618.94 µM × 100% = 46.919%