Overview
The research was done to assess the impacts of edge effects, forest age, and invertebrate exclusion on decomposition rates in a newly planted forest. The study found out that there were no correlations and interactions amongst the parameters that the research undertook to establish. The parameters used in the research were mean mass remaining, edge of the forest, age of the forest, and the abundance of the invertebrate organisms. The research method involved the use of three transects which each had six notches marked at 10 meters intervals and acted as data collection points. The data collected on various parameters were analyzed and results generated.
Introduction
To better understand the rate of decomposition, it is important to understand what decomposition generally means. When organisms such as animals, vegetations, insects, and other living organisms die, their bodies get broken down into several tiny pieces. The process by which the dead organisms are broken down into pieces either through mechanical or chemical means is referred to as decomposition (Swift, 1979, p.1). The process involves the activities of bacteria, certain kinds of worms and this may also involve some chemicals. The organisms involved in the process of decomposition are known as decomposers (Bharatdwaj, 2006, p. 43). The decomposers do not perform the task of decomposing the dead organisms as their primary goal, but the process is a way of their ingestion of food. The process of them ingesting dead bodies is what is referred to as decomposition (The University of Michigan, 1971, 72).
The decomposers depend on the dead organisms to develop, grow and have life sustenance (JSTOR 1956, p. 25). It is important to note that even the decomposers die and also eaten by other living organisms like bacteria (Limnological Society of Southern Africa, 1986, p.96). This means that the decomposers also get decomposed after they die. The implication is that decomposition is a cycle that will never end; when the decomposers breed they bring to existence new decomposers. After a complete decomposition, the remaining particles of the dead organism integrate into the ground and become part of the soil.
The rate of decomposition of forest litter coupled with the formation of various kinds of humus is greatly dependent on numerous factors. One of the most important of these factors is the climate. In the rate of decomposition is slowed in a cool climatic condition and in many cases, there can be found accumulation of remains of dead organisms in on the forest floor. Cool condition can exists due thick canopy which prevents the solar rays from reaching the forest floors hence is also a factor determining the rate of decomposition. The process of decomposition also depends on such factors as edaphic conditions and the quality of the soil fauna and the forest litter. The significance of each of these factors is divergent in terms of spatial and sequential scale (Agricultural Institute of Canada, 2008, pp. 443-867).
Literature Review
The process of decomposition is one of the most important processes of the ecosystem. Through decomposition nutrients go back into the soil to be used by plants in the manufacture of new food (Ecological Society of Australia, 1978, pp. 15-27). The plants are in turn food to many living organisms. Ecologists have argued that for new organisms to be born and survive the old one ones must die and decay. It therefore means that death and decomposition are integral part of the sustenance of the ecosystem. Through scientific study, it has been proven that decomposition of dead organisms is facilitated by decomposers (Gowariker, 2009, p. 181). The decomposers include, but not limited to, bacteria, some types of earthworms and insects. It therefore means that the rate of decomposition is a factor of the decomposers; the rate increases when the number of decomposers increases. Decomposers like bacteria increase in number when conditions such as hot humid conditions exist. Research literature indicates that forest experiencing hot humid conditions experience availability of large numbers of decomposers hence rate of decomposition in such forests are high.
Ecological scientific researchers have distinguished two types of decomposition: aerobic decomposition require oxygen while anaerobic decomposition takes place in the absence of oxygen. In view of this, aerobic decomposition can only be facilitated by the decomposers that require oxygen for their physiological functions. While anaerobic decomposition requires decomposers that can survive even in the absence of oxygen (Society for Applied Bacteriology, 1992, p.9).
Available literature also cites the importance of moisture in the process of decomposition. For faster rate of decomposition, the amount of moisture in the decomposing organism of heap must be as high as possible and should allow free infiltration of air so as to benefit aerobic bacteria. Research findings also indicate that the minimum amount of moisture that should support bacterial activities in the decomposition process should be around 12% to 15% (Martin, 1992, p.33).
Scientific studies have proven that temperature is such a vital part of decomposition. The findings argue that low temperatures during winter season slow down the rate of decomposition while high temperatures facilitates the rate of decomposition and this happens mostly during summer. The microbes known to be responsible for decomposition of raw organic substances are basically categorized into two: mesospheric microbes live and grow in temperatures ranging from 10° Celsius to 46° Celsius and thermophillic microbes live and grow in temperatures ranging from 46° Celsius to 70° Celsius. Ecologists have identified that decomposition taking place at high temperatures is of great benefit to gardeners since the temperatures kill germs and weed seeds that may be dangerous to the vegetables. Earthworm lives in the soil and its activities are very vital to decomposition of dead organisms or heap. Its barrowing tendency helps to create spaces that make aeration within the decomposing heap possible. Earthworms also derive their food from the decomposing organic matter and hence facilitate decomposition in the process (Consultants Bureau, 1972, p.317).
Research studies have also proven that the rate at which decomposition take places in within the forest soil depends on exposure to open air, moisture and water. The findings reveal that organic matters that are placed underground take more time to decompose than those exposed on top of the soil. The findings further state that the rate of decomposition reduces with the depth of burial of organic matter. This, the researchers say, can be explained by reduced exposure to air or oxygen; the deeper the burial of decomposing organic matter, the less the availability of air to facilitate decomposition and the more time taken for complete decomposition (National Research Council of Canada, 1987, p.2030).
Insects have also been found to play the role of decomposers. They are specifically important for decomposition of organic matters that are found on the surface of the earth within the forest. Just like other decomposers, insects also derive some of their food from the dead or cheap or organic matter. Amongst the catalysts of decomposition is light. Light from the sun is a great source of heat energy that is necessary for decomposition; it also provides the plants with energy for photosynthesis within the forest. It is important that note that the availability of light to forest soil is determined by the canopy layers formed by the trees above. The more the canopy layers the less the amount of light reaching the floor of the forest (Mullen, 2009, P.40).
Critical studies that have been conducted have revealed that de composition in the forests is an important stage in the nutrient cycle. Through decomposition nutrients are taken back into the soil to be used by trees again in the manufacture of new food through photosynthesis. The tree leaves are eaten by both micro-organisms and the invertebrates found within the forest (Pacific Northwest Research Station, Nd, p. 74). So it is natural that the invertebrates are naturally attracted to the decomposition sites within the forest by the availability of food materials, which are the fallen leaves from the forest trees. Research literatures further indicates that decomposition process is facilitated differently by the decomposers. The process is commenced by the bacterial activities then the rest follow and then the process continues to the point where the remnants are integrated into the soil as parts of the soil (Tasmania, 1939, p. 14). The role of the invertebrates in the decomposition process is to sectionalize the litter into smaller pieces and also mixing the litter with the mineral soil hence divulging a wider surface area for microbial colonization (Bloem, 2006, p.25).
Problem Statement
There is a great importance to the study of decomposition of forest soil with regard to its role in maintaining the ecosystem. The research studies that have always been done have not revealed the nature of decomposition that take place within the Motutapu and scrubland soils. It can be argued that decomposition of organic matter is the same in ecosystems experiencing similar climatic conditions coupled with the availability of necessary decomposers. However, it is important to note that different ecosystems experience different climatic conditions. The distribution of the decomposers also varies from one ecosystem to the other depending on the availability of necessary conditions for survival. There is a difference of decomposition in old forests and the new ones. It is believed that there are impacts of edge effects, forest age and invertebrate exclusion on decomposition rates in a newly planted forest. It is therefore important to study the Motutapu and scrubland soils to determine whether this is the case. The research question that was during the research study therefore was, “Is it true that there are impacts of edge effects, forest age and invertebrate exclusion on decomposition rates in a newly planted forest?”
Research Objectives
The main objective of the research was to find out if there are impacts of edge effects, forest age and invertebrate exclusion on decomposition rates in a newly planted forest.
Hypotheses
- Decomposition rate will increase as forest ages;
- The rate of decomposition increases with distance from the edge of the replanted forests; and
- When the size of invertebrates that is able to access litter increases, the rate of decomposition also increases.
Methodology
Three transects of 60 meters each in length 50 meters from the upper age of the replanted forest and continued toward the centre of the forest. Each of the three transects had six notches each marked at intervals of 10 meters. The notches served as data collection points. The first transect was located within the trees that were 8 years old, the second one was placed through trees of ten years old and the last transect was located within trees that were fifteen years of age. Litter bags of two mesh sizes measuring 1mm and 5mm was each placed at ten-metre interval of all transects except the first one; there was high possibility that the tall and thick grass coverage present with that study area would most likely cause unprecedented damages to the litterbags and adversely obstruct significant data collection. Each of the 36 litterbags contained ten mature freshly pulled out leaves.
Moreover, five more standardized litter bags were filled with other leaves collected from the same region the same day; the mass of the leaves were determined after which they were laced in oven to dry at 70° Celsius for a period of 24 hours. Thereafter the dry weight measurement of the leaves was obtained. The 36 litterbags were retrieved after a two-month period; they were dried and then their contents weight again and then the dry weight that was lost each litterbag was calculated and the percentage remaining mass of the contents was established.
Ambiotic measurement was also obtained from transect points, a light meter was used to measure the light intensity reaching the ground level, soil temperature and moisture was measured by use of a probe and the pH of the soil that lies immediately beneath the litter layer was examined. The litter within 25mm2 quadrat around the litterbag was weighed; invertebrates were juddered from it and the numerically determined to in order to approximate the mega and macro-faunal abundance in each of the regions. A point centred quarter stick was placed at each transect point and the species of the four nearest trees of greater than 15cm in height was recorded.
Data Analysis
The data analysis process used the R commanders for two-way ANOVA for fine and coarse against age; fine/coarse against Location, and Location against Age. For other mystifying factors, a multiple regression was done.
Results
The table 2 below indicates p-values from ANOVA outputs from ‘R’ for mass remaining for: A – mesh type, age, mesh type and age interaction, B – mesh type and age, C- mesh type.

The result obtained from the study, as shown in the table2 above indicated that there was no interaction for the mass remaining between mesh type and age. It also indicated that there is no difference in the mean mass remaining between the different ages of the sites. However, it indicates that there is a significant difference in the mass remaining between the different mesh types. In other words, table two shows that with 95 percent confidence there was no interaction between mesh type and the location on thee transect (p-value=0.13107). There was also no evidence against the null hypothesis that the underlying means for the location on thee transect (edge, middle and centre) are the same (p-value=0.20098). And lastly there was evidence against the null hypothesis that the underlying means for mesh type are the same (p-value=0.00158).
The table 3 below shows the p-values from ANOVA outputs from ‘R’ for Mass remaining for: A – age, location, age and location interaction, B – age and location.
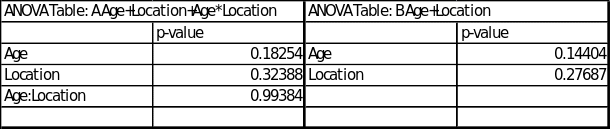
According to the table it is evident that there is no interaction for the mass remaining between mesh type and location, the table also shows that there is no difference in the mass remaining means between the different locations on the transect and there is no interaction for the mass remaining between age and location. In other words, the table indicates that with 95 percent confidence there was no interaction between the age of the site and the location on thee transect (p-value=0.99384). There was also no evidence against the null hypothesis that the underlying means for the age of the site (old, middle and new) are the same (p-value=0.14404). Lastly there was no evidence against the null hypothesis that the underlying means for the location on thee transect (edge, middle and centre) are the same (p-value=0.27687).
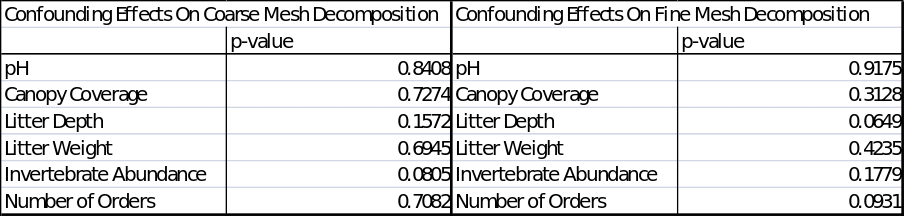
As for the confounding factors, the only difference in the mean mass remaining, occurred between the fine and coarse mesh decomposition bags, therefore, a multiple regression analysis was to be completed to observe if there was an impact on the mass remaining by other factors such as pH, canopy coverage, litter depth, litter weight, invertebrate abundance and the number of orders. The results of the multiple regression is summarised as in the table below:
P-values from multiple regression outputs from ‘R’ for mass remaining for coarse and fine mesh.
The following are the trends in times and space according to the research outcome:
- Invertebrate abundance typically greatest at edges and in young forest.
- Invertebrate diversity typically greatest at edge.
- Increase in litter weight with time.
- Increase in canopy cover with time.
- Decrease in pH with time.
Discussion
After the whole process of research we found out that, there is no significant difference that exists between the rate of decomposition and the age of the forest. It also came out clear that there is no considerable difference in the rate of decomposition in relation to the edge, middle and the centres of the used transects taking into account both within a transect and between one transect and the other. This is in consistence with the other research findings indicating that there is no significant change in the rate at which leaf-litter decomposition took place as compared from the interior to outward edges of the forest. It is almost impossible to explain the insignificant edge difference.
The research study also found out that the rate at which decomposition took place was not correlated with the difference in air temperature, the depth of litter, moisture contents, and densities of invertebrates. These findings were in relation to the edge of transects and across all the sites used during the study. This finding is in contrast with other research findings indicating that edge difference affects the rate at which decomposition takes place. However, the removal of some lea-litter by the feeding termites is the cause for the effects of edge difference.
There was also a significant variation between the mass remaining in the coarse and fine litterbags. The greater mass that was remaining in the fine bags shows that the rates of decomposition increase when larger invertebrates are able to access the litter, which is in consistence with other research findings. We also found out the presence of mesofauna, microfauna and macrofauna has an impact on decomposition within a restored NZ forest system.
Reference List
Agricultural Institute of Canada. (2008). Canadian journal of soil science, Volume 88, Pages 443-867. Canadian Society of Soil Science, pp. 443-867.
Bharatdwaj, K. (2006). Physical Geography. Biogeography, Discovery Publishing Houses, P.43.
Bloem, J. et al. (2006). Microbiological methods for assessing soil quality. CABI Publishing Series. United Kingdom: CABI, P.25.
Consultants Bureau. (1972). The Soviet journal of ecology, Volume 2. Consultants Bureau, p.317.
Ecological Society of Australia. (1978). Australian journal of ecology, Volume 3, Issues 2-4. The University of California, pp. 15-17.
Gowariker, V. (2009). The Fertilizer Encyclopedia. New York: John Wiley and Sons, p.181.
JSTOR (Organization). (1953). Quarterly review of biology electronic edition, Volume 28. University of Chicago Press. Journals Division, University of Chicago Press for the State University of New York at Stony Brook, p.25.
Limnological Society of Southern Africa. (1986). Journal of the Limnological Society of Southern Africa, Volumes 12-14. Limnological Society of Southern Africa, p. 96.
Martin, D. (1992). The Rodale book of composting. Rodale, p.33.
Mullen, G. (2009). Medical and Veterinary Entomology. Academic Press, P.40.
National Research Council of Canada. (1987). Journal canadien de botanique, Volume 65, Issues 9-12, p. 2030.
Pacific Northwest Research Station. (Nd). Fall River long-term site productivity study in coastal Washington site characteristics, methods, and biomass and carbon and nitrogen stores before and after harvest, p.74.
Society for Applied Bacteriology. (1992). The Journal of applied bacteriology, Volume 72. Symposium series. Blackwell Scientific, p.9.
Swift, J. et al. (1979). Decomposition in terrestrial ecosystems, Volume 1979, Part 2. Volume 5 of Oakland Project Series. United States: University of California Press. pp. 1-12.
Tasmania. (1939). The Tasmanian journal of agriculture, Volumes 8-9. Tasmanian Dept. of Agriculture, p.14.
The University of Michigan. (1971). Journal of theoretical biology, Volume 30. Academic Press, p.72.