Nitrite Ion and Indole
Nitrite ion (NO2−) is a chemical compound consisting of one nitrogen atom covalently attached to two oxygen atoms. It is produced as an intermediate in the oxidation pathway from nitric oxide (NO) radical to the stable metabolite nitrate (NO3−). The nitrate ion can undergo protonation to form nitrous acid, which is an unstable, weak acid that is highly volatile. This ion can also undergo oxidation and reduction reactions depending on the strength of the reducing or oxidizing agent. The interaction of nitrate ions is unique such that it can bind in more than one way. Ions that exhibit this phenomenon are known as ambidentate (César et al. 15582). Nitrite ion is capable of attaching itself to metal centers in five different ways.
The formation of nitrite ion is a crucial step in the nitrogen cycle. In the air, nitrogen oxides can be transformed into nitrite ions, which combine with atmospheric moisture to form acid rains (Singh et al. 294). The concentration of nitrate ions in chemical and biological systems is very important due to its impact on physiological reactions. Nitrite ions are toxic in high concentrations inside living organisms because they can oxidize hemoglobin to methemoglobin, thus interfering with the ability to convey oxygen in red blood cells.
Microorganisms are capable of oxidizing or reducing ammonia and nitrates in organic compounds, thereby leading to the production of nitrites. Therefore, the presence of nitrite in water is usually used as an indication of pollution due to the formation of nitrite ions during the biodegradation of these compounds (Zheng et al. 315).
Furthermore, the incorporation of nitrite in foods (as a food preservative in cured meats), detergents, fertilizers, and other industrial applications has escalated the issue of pollution. From an environmental point of view, nitrite is known to play a vital role in the chemical reactions that involve secondary amines in the formation of N-nitrosamines, which are known to be strong carcinogens (De Mey et al. 2910). Therefore, the determination of this ion crucial from a health and pollution standpoint.
The U.S. Public Health Service reports that the maximum permissible threshold of nitrite in drinkable water is 0.06 ppm (Benson et al. 1643). As a result, the quantification of nitrite concentrations is crucial in the areas of food safety, diagnosis of diseases, control of water quality, forensic inquiries, and fundamental biological studies involving the physiology and pathology of nitric oxide.
Indole is an organic compound with a chemical formula of C8H7N. The presence of the nitrogen atom implies that it is an amine. It has a ring-like structure consisting of two rings and is classified as an aromatic heterocyclic compound. The first ring is a six-sided benzene ring that is joined to a five-sided pyrrole ring. Indole is widespread in the natural environment and has a characteristic fecal odor in high concentrations.
Nonetheless, at low concentrations, it produces a flowery smell, which makes it a component of flower scents and perfumes. Microorganisms can produce it following the enzymatic breakdown of the amino acid tryptophan. This compound serves signaling purposes in bacteria and is responsible for various physiological processes in bacteria, for example, the formation of spores, drug resistance, virulence, the stability of plasmids, and formation of a biofilm.
Indole detection, on the other hand, is important in different microbiological tests that aim at detecting specific microorganisms. The chemical reaction between nitrites and indole usually leads to the formation of a compound with a pinkish to red color. The intensity of the color is usually a reflection of the product concentration and can be used to determine the concentrations of the original reactants.
Indole goes through electrophilic substitution at the third carbon position, thereby leading to the formation of numerous synthetic precursors. This position is preferred due to its high reactivity, which is reported to be about 1013 times higher than benzene. Given the reactivity of C3, subsequent electrophilic substitutions can only occur after substation reactions have taken place at C3, C2, and N1. However, in acidic conditions, the C3 position can be protonated adequately thus leading to the preferential electrophilic substitution at the fifth carbon position.
Measuring Nitrate Ions
Most techniques used to assay nitrite rely on spectrophotometry due to its reported simplicity and reliability. These methods can be grouped into two major classes known as type 1 and type 2. Type 2 reactions are founded on Griess modification, whereas type 2 reactions use nitrosation reactions as their basis. Nitrosation reactions, which are achieved by nitrite esters or nitrous acids, have been studied for more than 10 decades.
Typical nitrosation reactions occur in pyrimidines possessing either of the three electron-donating moieties such as thioxo, oxo, or amino groups. In pyrimidines with two substituted groups, the comparative position of the substituted groups determines the likely reaction. As such, nitrosation at the 5th position can occur in 4,6-diamino- and 4,6-dihydroxypyrimidines, whereas no reaction can take place in their 2,4-isomer. Various analytical protocols have been developed based on these major groups with type 1 reactions having more methods compared to type 2.
The Griess reaction entails the formation of an azo dye through a diazotization reaction between an aromatic amine and nitrite, followed by joining of the diazonium cation with other aromatic amines or phenols (Deng et al. 152). This method was named after a German organic chemist Johann Peter Griess, who demonstrated the initial diazotization of arylamines. This reaction later formed the major reaction in the production of azo dyes that are commonly used in the contemporary dye industry. The key chemical reagent used comprised a mixture of sulfanilic acid and α-naphthylamine in dilute sulfuric acid (Deng et al. 152). Figure 1 shows a schematic illustration of the Griess reaction.
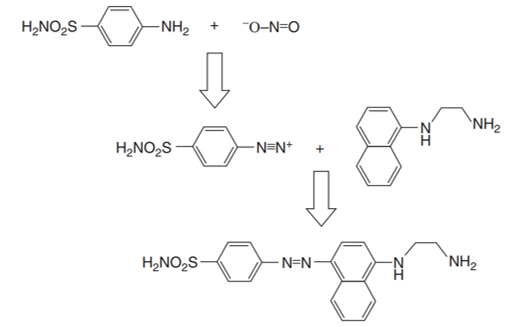
Most of the techniques based on the Griess reaction provide appreciable levels of sensitivity and selectivity. However, they require stringent measures to regulate temperatures and the temperatures and pH in the course of the diazotization step.
Furthermore, they also need prolonged coupling time. Some studies have attempted to use p-aminophenyl mercapto acetic acid as a diazotizable amine alongside coupling agents such as 8-hydroxyquinoline and alpha-naphthol to determine nitrite ions using spectrophotometry. Even though these attempts produced very fast diazotization and coupling reactions, the level of sensitivity was reduced, and the methods were highly predisposed to interferences in the absence of masking agents.
The use of NED as a coupling agent was advantageous because it combines in an acidic medium. On the other hand, p-aminophenyl mercapto acetic acid possesses a highly reactive amino group, a carboxyl moiety, and a mercapto group that allows it to link up with metal ions in various polydentate styles. This reaction is thought to promote the chelation of these ions in solution, leading to an increase in the tolerance bounds for these ions in the analyte. Common ions that contribute to interferences in other methods include sulfite, copper II, iron III. However, this experimental approach circumvents these interferences.
The Griess reaction has undergone various modifications over time to determine the viability of simple reagents in the spectrophotometric detection of nitrite ions using indole. One such method entails adding an indole solution to the test sample followed by acid, topping up with distilled water where necessary, and allowing the mixture to stand for 10 minutes before measuring the resultant absorbance at 530 nm.
A blank solution containing the acid, distilled water, and indole is used as the reference solution. A calibration curve is then prepared by finding the absorbance of different reaction mixtures containing varying quantities of nitrite ions. Plotting the absorbance versus concentration should produce a straight line passing through the origin to show that the assay abides by the Beer-Lambert law. This calibration curve can then be used to determine the concentration of a sample with an unknown concentration of nitrite ions.
To enhance the accuracy and precision of this experimental approach, the impact of various parameters has been investigated. For example, the effect of indole on the peak production of the colored complex by adding various volumes of 0.05% indole reagent to the reaction mixture. It was observed that optimal production was attained when the concentrations of the indole reagent ranged from 0.8 to 1.0 ml. Larger volumes of the indole reagent were associated with a 20 to 40% reduction in absorbance.
The impact of mineral acid on the reaction has also been studied. About 5 different acids have been tested, including hydrochloric, acetic, sulfuric, nitric, and phosphoric acids. Maximal intensities have been realized with nitric and hydrochloric acids. However, nitric acid results in high absorbance values in the blanks, which obscures the outcomes. Acetic acid only produces about 40% of the total intensity generated by hydrochloric acid, whereas sulfuric and phosphoric acid elicited 89% of the absorbance produced by hydrochloric acid. Therefore, hydrochloric acid is the preferred acid in the assay even though sulfuric acid and phosphoric acids can still be used.
The impact of the sequence of reagent addition and the stability of the resultant color. To obtain maximal absorbance, the indole solution should be added to the test sample (anticipated to contain nitrite) followed by the indole solution and finally the acid solution. Changing the order of reagent addition is reported to reduce the overall absorbance by approximately 6% (Wang et al. 712). Even though a color change occurs immediately after the reagents have been mixed, it is necessary to allow the mixture to settle for about 10 minutes to obtain the maximal absorbance. The absorbance remains constant for about 45 minutes, after which it begins reducing. Therefore, under experimental conditions, researchers ought to ensure that absorbance readings are taken within this duration.
Another recommended method for the quantification of nitrites is the 2,3-diaminonaphthalene (DAN) assay that uses fluorometry (Wang et al. 711). However, this method results in slow detection because nucleophiles are deactivated in highly acidic media. Furthermore, this method requires very long incubation times to permit the completion of the reactions, which lowers the detection throughput.
The reaction of nitrite with sulfanilamide and NED at different pH values has been investigated. Sodium nitrite was used as the nitrite source at concentrations ranging from 2 to 30 mM. The salt was incubated with Griess reagent consisting of 1% sulfanilamide and 0.1% NED made using different inorganic acids to make solutions whose pH ranged from 0.6 to 3.3. It was noted that using solutions with low pH values resulted in solutions with reduced final absorbance. Inadequate incubation time was ruled out as the cause of the low absorbance because repeating the absorbance readings after 30 minutes did not produce substantial increases in absorbance.
These findings showed that elevated pH ranging from 2.5 to 3 was preferred in the reaction because it resulted in higher responses. However, apart from its use in assays involving water samples, the Griess reaction is sometimes applied in biological samples that may contain additional molecules that could interfere with the assay. Therefore, it may be necessary to play around with different parameters when dealing with biological fluids to determine combinations and concentrations that yield optimal responses (Giustarini et al. 367).
The chemical reaction between nitrite and indole is also affected by the type of sample being tested. For example, biological samples often present additional impurities that may contribute to interference. Therefore, blood samples may require additional processing steps before subjecting them to analysis. In practice, human blood can be kept for one hour at 37oC followed by centrifugation at 15,000g for 10 seconds to obtain nitrite-free plasma (Giustarini et al. 365).
Principles of Spectrophotometry
Spectrophotometry is a chemical technique that measures the concentration of analytes based on the amount of light that is absorbed in a given wavelength when white light passes through a cuvette containing the test solution. The fundamental principle of spectrophotometry is that each substance is capable of absorbing or reflecting light within a given range of wavelengths.
Spectrophotometry has wide applications in various fields, including physics, chemistry, biochemistry, clinical uses, industries, as well as material and chemical engineering. The rate of enzyme-catalyzed reactions as well as the concentrations of the resultant products can be determined using spectrophotometry in biochemical applications. Clinical diagnoses can benefit from spectrophotometry through the examination of blood, tissues, and other body fluids such as urine and sweat.
The basic instrument used in spectrophotometry is a spectrophotometer, which is an appliance that quantifies the intensity of light that is absorbed after passing through a solution. This way, the concentration of a known chemical substance can also be ascertained by quantifying the amount of light that has been absorbed. Spectrophotometers can be grouped into two based on the range of light wavelength emitted by the light source: ultraviolet (UV)-visible spectrophotometer and infrared (IR) spectrophotometer.
The UV-visible format makes of light rays within the ultraviolet range and visible light range, which are 185 to 400 nm and 400 to 700 nm of the electromagnetic radiation spectrum, respectively (Germer et al. 244). Conversely, the IR spectrophotometer exploits the infrared range that spans between 700 and 15,000 nm of the electromagnetic radiation spectrum.
A unique feature of visible spectrophotometry is that the observed color can be used to establish the absorption or the transmission of a given substance. For example, it is expected that a solution absorbs light throughout the visible range and does not transmit at any of the visible wavelengths should appear black. Conversely, solutions that transmit all visible wavelengths devoid of any absorption should appear white to the eye. Similarly, substances that take in red light whose wavelength is approximately 700 nm appear green to the eye given that green appears opposite red on the color wheel (complementary colors). Practical operations of the visible spectrophotometer involve the use of a prism to sieve other light wavelengths to allow only light at a specified wavelength to traverse a solution.
A spectrophotometer is a hybrid of two gadgets namely a spectrometer and a photometer (Germer et al. 240). A spectrometer can be defined as an instrument that generates, disperses, and quantifies light. The needed wavelength of light is produced and passed to a collimator, which is a lens that conveys a straight light beam that traverses a monochromator, a glass prism that breaks up the light into diverse wavelengths. At this point, a wavelength picker conveys only a specific wavelength as chosen. This light is then allowed to pass through a sample in a cuvette. In contrast, a photometer is a photoelectric detector whose purpose is to quantify the strength of light beams. It senses the amount of light that has been taken in by the sample and relays this information to a digital display.
The quantity of light that traverses the cuvette into the detector is influenced by the length of the cuvette as well as the concentration of the sample. Also, the quantity of light that passes through the cuvette can be related to the intensity of the original light beam in what is known as the transmittance. Therefore, transmittance can be defined as the proportion of light that traverses the sample in the cuvette and can be computed using the equation Transmittance (T) =It/Io where It is the intensity of light following the passage of the original beam through the cuvette and Io is the initial intensity of the light beam before passing through the cuvette.
Conversely, Absorbance (A) can be associated with the transmittance using the equation Absorbance (A) = -log(T) = -log (It/Io). In this equation, absorbance indicates the quantity of light that has been taken in. If the absorbance value is known in the above equation, it is possible to find the concentration of a sample using the Beer-Lambert Law, which implies that a linear association exists between the concentration of a sample and its absorbance.
This relationship can be expressed as A= ϵlc where A is the absorbance, ϵ is the molar absorptivity, l is the path length in centimeters, and c is the concentration of the sample (Germer et al. 247). The molar absorptivity is often expressed as a constant that differs from one substance to another. Its units are L·mol-1·cm-1. For these reasons, the units ought to cancel out during the calculation to give the concentration of a substance in moles per liter.
Objective and Hypothesis
The objective of this study was to determine the impact of various reagent concentrations in the reaction between nitrite and indole in propanol and sulfuric acid to form a red-colored compound. It was hypothesized that increasing the concentrations of the reagents would affect the color intensity of the product. Various concentrations of nitrite, indole, sulfuric acid, and propanol were used in the experimental procedure.
Works Cited
Benson, Robert, et al. “Human Health Screening and Public Health Significance of Contaminants of Emerging Concern Detected in Public Water Supplies.” Science of the Total Environment, vol. 579, 2017, pp. 1643-1648.
César, Vincent, et al. “Homo-and Heteropolymetallic Complexes of the Hybrid, Ambidentate N-Heterocyclic Carbene Ligand IMes-acac.” ACS Omega, vol. 3, no. 11, 2018, pp. 15582-15591.
De Mey, Eveline, et al. “Volatile N-nitrosamines in Meat Products: Potential Precursors, Influence of Processing, and Mitigation Strategies.” Critical Reviews in Food Science and Nutrition, vol. 57, no. 13, 2017, pp. 2909-2923.
Deng, Tao, et al. “A Novel Strategy for Colorimetric Detection of Hydroxyl Radicals Based on a Modified Griess Test.” Talanta, vol. 195, 2019, pp. 152-157.
Germer, Thomas A., et al. Spectrophotometry: Accurate Measurement of Optical Properties of Materials. Elsevier, 2014.
Giustarini, Daniela, et al. “Nitrite and Nitrate Measurement by Griess Reagent in Human Plasma: Evaluation of Interferences and Standardization.” Methods in Enzymology, vol. 440, 2008, pp. 361-380.
Singh, Shweta, et al. “Rain pH Estimation Based on the Particulate Matter Pollutants and Wet Deposition Study.” Science of the Total Environment, vol. 563, 2016, pp. 293-301.
Wang, Qiu-Hua, et al. “Methods for the Detection and Determination of Nitrite and Nitrate: A Review.” Talanta, vol. 165, 2017, pp. 709-720.
Zheng, Xiang-Juan, et al. “One-Step, Stabilizer-Free and Green Synthesis of Cu Nanoclusters as Fluorescent Probes for Sensitive and Selective Detection of Nitrite Ions.” Sensors and Actuators B: Chemical, vol. 230, 2016, pp. 314-319.