Instrumental methods are used for qualitative chemical analysis, many of which are represented by spectroscopy. The general idea of spectroscopic observations is reduced to the excitation of molecules by various external factors — usually an electromagnetic field — to study the characteristic bands or peaks in the analytical signal output of the substance. Most spectroscopic experiments produce unique signals from which the analyzed substance can be detected with high accuracy. One such technique is nuclear magnetic resonance on proton nuclei (1H NMR), which studies the reorientation of magnetic moments of nuclei with non-zero spin. The 1H NMR output signal is a spectrogram with labeled peaks that correlate with the transitions of magnetic nuclei between different energy states. With 1H NMR, it becomes possible to determine tacticity in polymers; in general, tacticity should be understood as the unique way in which side groups are arranged in an organic molecule, which is especially relevant for macromolecular chains.
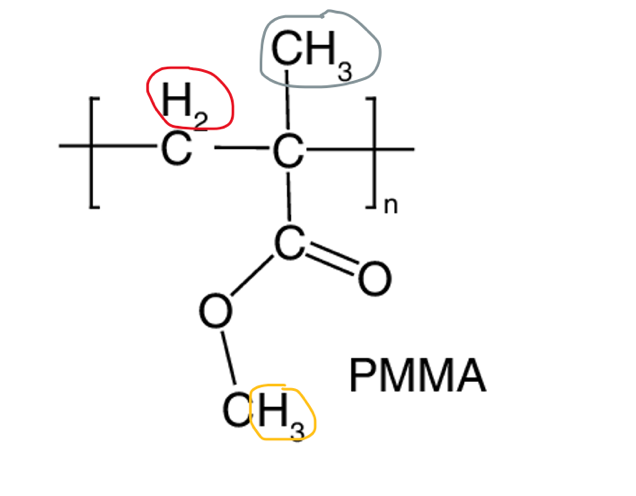
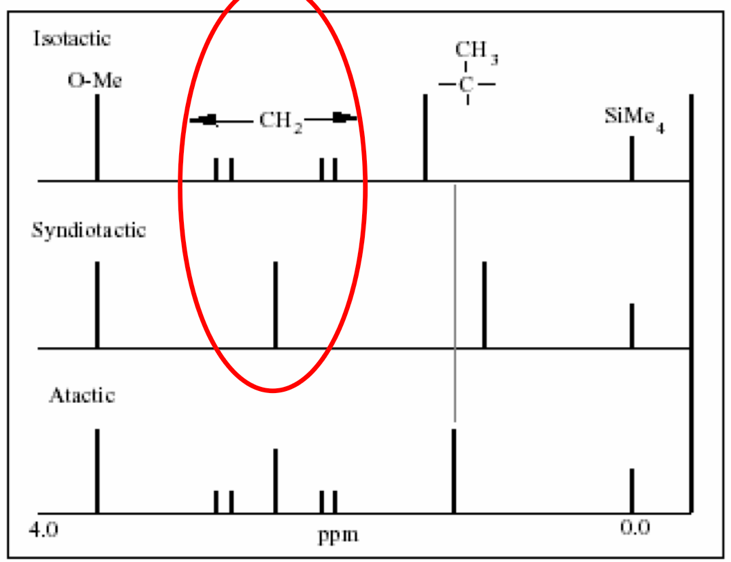
To specify the discussion, the essay will use polymethyl methacrylate (Figure 1) to demonstrate the ability of 1H NMR to detect the side groups of this molecule. The side chain fragments, in this case, are hydrogen, a methyl group, and -COOCH3, located outside the plane of the central carbon bond. It should be understood that the methylene group proton (red) is bound to three neighboring protons, which creates a quartet signal cleavage; the gray proton bound will give a quintet cleavage, and the yellow group proton in -COOCH3 has no neighboring hydrogen — singlet. Notably, the stoichiometric arrangement of these groups affects the tactile shape of the molecule and creates three different 1H NMR signals: isotactic, syndiotactic, and atactic. In the case of isotactic orientation, when the methyl groups of the polymer side chain are on one side, four bands appear in the spectrum at the methylene group due to the influence of -CH2. (Figure 2). However, in the case of the syndiotactic arrangement, in which the methyl groups alternate, the neighborhood is inhibited, resulting in the appearance of one line in the center of the previous four. Interestingly, if the polymer is atactic, it proves impossible to separate isotactic and syndiotactic polymers because both quintet and singlet bands appear simultaneously. The methyl group gives a singlet cleavage instead of the expected quintet cleavage because -CH2 in the polymer is surrounded by the same neighbors, which makes these hydrocarbons equivalent, and therefore only singlet is observed. Thus, the 1H NMR can be used results to determine the tactility of the polymer.
References
Wood-Adams, P. M. (n.d.). Characterization of polymers by NMR [PDF document]. Web.