Introduction
Opioids are classified as a classification of drugs that are used to suppress pain through binding of the Opioid receptors which form are located the gastrointestinal channel and the central nervous system. The opioid receptors in these two organs transmit both the positive outcomes and the negative effects of these substances. The analgesic effect of Opioid substances is achieved through decreasing the levels of pain detection; boosting the ability to tolerate pain and reducing the reaction to the perception of pain. By imposing these three effects these substances help reduce and; or relieve the levels of pain experienced by individuals. (Campbell, 2003)
The negative effects of opioids include constipation; respiratory difficulties and feelings of being sedated. Other effects of the use of Opioid substances include cough repression that can be an indication of either Opioid substance use or an unexpected outcome after administration. Opioid administration can also lead to physical reliance that brings about withdrawal syndrome after discontinuation; and feelings of Euphoria. Further the over dosage of these drugs is capable of bringing about death among other adverse effects. (Beers & Robert, 2002)
The focus of this work is to state and explain the grouping of Opioid drugs based on the chemical and pharmacological grouping. The drugs in question will be analyzed based on their chemical classes, and characteristic features like acid-base properties; structural features; solubility; polarity; ionization properties; partition and metabolism rate and channels. These include Fentanyl C22H28N2O ; Methadone C21H27NO; Loperamide C18H21NO3 ; Propoxyphene C22H29NO2; Naloxone C19H21NO4; and Pentazocine. (Kenneth, 2002)
Drug classification, chemical grouping and characteristics
When looking at the classification of opioids; six classes are distinctive and these include the endogenous opioids that are formed naturally by and within the body. An example of these is enkephalins. Another classification of opioids is the semis-synthetic that are formed from the use of natural opiates and an example of these is the hydromorphone. The other classification of these substances is the fully synthetic opioids such as pethidine; and the other classification is that of natural opiates that exist naturally in the resin of the opium poppy. (Beers & Robert, 2002)
As discussed earlier the main aim of these drugs is to influence the working of receptors that detect and communicate the effect of pain to the brain. Further drugs are referred to as acidic when they dissolve to donate a proton or basic when they donate at least one electron that is neutralized by a proton. However the drugs that exist in non-acidic or basic functional groupings are given the name non-electrolytes. In this sense it can be noted that the drugs that are basic like Methadone that have a higher pKa value are more likely to ionization as they are strong bases. The acidic surroundings needed for the ionization process to take place include the stomach. (Campbell, 2003)
The absorption scheme and channel for the different Opioid drugs is however, dictated by the method or area of administration used. These administration methods include: Oral administration; rectal administration; intramuscular administration and intravenous administration. The pathways used for the absorption of the different Opioids are common and one is shown in the diagram.
Drug Metabolism factors
After the reaction or absorption of drugs is when the metabolism rate of a drug is observed and this is where lipophilic compounds are converted into regular polar products. This rate is used in determining the intensity and duration of the drug on the body, and is mainly dictated by the ability of the liver to absorb the drug through chemical reactions. (Kenneth, 2002)
Acid-base properties.
Drug review
Fentanyl
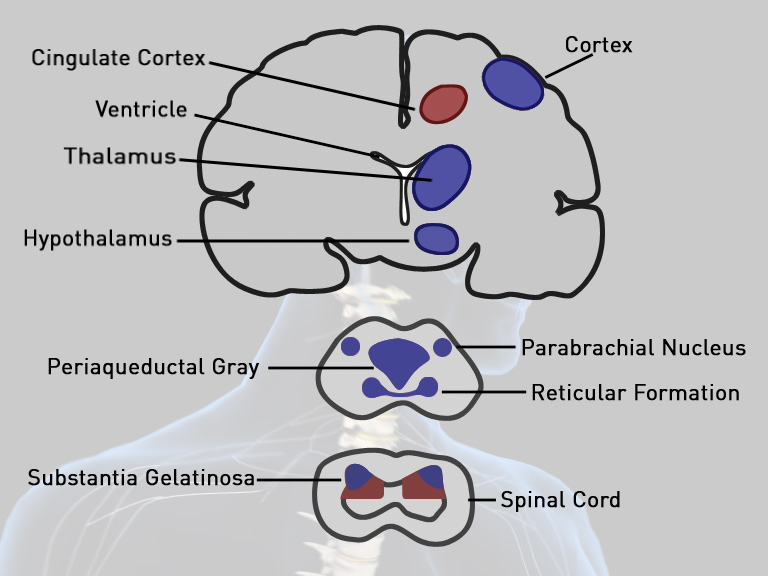
The chemical formula of its molecules is represented in figure below.
The molecular mass is 336.47 g/mol. This drug is used on cancer patients with intensive pain that is not addressed by the regular narcotic therapy. This drug works through acting on the Opioid mu-receptors which are located in the brain and the spinal cord. Its primary medical use is for analgesia or sedation through increasing the individuals’ tolerance to pain and the perception of it. It also does work through alteration of mood; drowsiness; or causing dysphoria. Fentanyl works through its conversion to morphine where opioids shut N-type run calcium communication (OP2-receptive agonist) and open calcium-dependent internally correcting potassium channels (OP3 & OP1 receptive agonist). This brings about hyper polarization thus reduced neuronal excitability. (Compton & Athanasos, 2003)
On the question of absorption Fentanyl has a bioavailability of 92% when administered transdermally and 50% when administered buccally.This drug is metabolized mainly through human cytochrome P450 3A4 isoenzyme structure and has a half-life ranging from 3-12 hours. The drug normally exists in a solid state and has a water solubility of 200mg/l. (Kenneth, 2002)
On the area of conjugate acid-base pairing, Fentanyl being a weak acid reacts as an H+ ion donor thus forming a conjugate base. Fentanyl hydrolysis led to the formation of despropionylfentanyl and alkyl hydroxylation led to the formation of hydroxyfentanyl. Fentanyl ionization takes place through its donation of an H+ particle depending on the receptor characteristics that are either acidic or basic. This process further leads to neutralization when the reacting agents are basic and this leads to the formation of a salt and water. (Beers & Robert, 2002)
Methadone
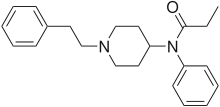
The chemical formula for the drugs molecule is shown above. This drug as a hydrochloride and primarily acts like a mu-pied agonist and has similar use as that of morphine except that it also has a restraining effect on cough centre like the one associated with fatal lung cancer. It is also used as treatment for dependence on opioid drugs though lengthened use of it may cause the same. It has an average molecular weight of 309.44g/mol and exists in solid form and has a water solubility of around 03 mg/ml. This drug is used for relief of severe pain; and detoxification management of narcotic addictions but is more active and toxic than morphine. (Kenneth, 2002)
Methadone works through acting as an antagonist at the N-methyl-D-aspartate receptor. The absorption of methadone following oral administration varies between 36 and 100%. In cases of over dosage it may lead to death; circulatory failure; cardiac arrest and apnea. On biotransformation cytochrome P450 enzymes convert methadone to EDDP and other dormant metabolites that are excreted through the urinary system. This drug has a half-life of between 24-36 hours and works by acting on the target areas that include the mu-opioid receptor; glutamate receptor subunit 3A; and the neuronal receptor subunit alpha-10. (Compton & Athanasos, 2003)
On the area of conjugate acid-base pairing; Methadone being weak acid and possessing weak basic traits either reacts through donating a H+ ion or an A- ion forming a conjugate solution. Methadone hydrolysis is done using Tartaric acid in an acetone /water mixture forming dextro-methadone; levotartate and levo-methadone. Methadone ionization takes place through either the positive ion donation H+ or the negative functional group depending on the receptor characteristics that either accept or donate conjugating functional groups. This process brings about neutralization that leads to the formation of a salt and water. (Beers & Robert, 2002)
Loperamide
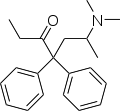
The chemical formula for the molecule is shown below.
This drug is a long duration antidiarrheals though not significantly absorbed from the gut. It however has no impact on the adrenergic structure or the nervous network but may be used to antagonize histamine and to alter acetylcholine release. It has a molecular mass of 476.22 g/mol; exists in solid form and has slight water solubility of 04mg/ml. This drug is used for the treatment of diarrhea associated with inflammation of the bowels or gastroenteritis.
Loperamide is an opioid receptor agonist that acts on the mu-opioid receptors within the large intestine. It does not affect the central nervous system but rather works by affecting the muscle system within the intestinal walls. By this process it increases fluid absorption; reduces colonic mass loss and restrains the gastrocolic reflex. In practical working it is a non-selective calcium blocker through binding to the mu-receptors; calmodulin and the NMDA receptors. (Compton & Athanasos, 2003)
On absorption, it’s not significantly absorbed from the gut; has a protein binding capability of 97% and overdose symptoms like constipation and nausea. This drug has a half-life of 9.1 -14.4 hours and is metabolized by the enzyme cytochrome P450 3A4 (CYP3A4) (Kenneth, 2002)
On the area of conjugate acid-base pairing; Loperamide being a weak acid reacts donating an H+ ion to form a conjugate base. Loperamide hydrolysis id done using carboxylesterases to form fluorouracil; and the ionization process takes place through its donation of an H+ ion leading to neutralization that forms a salt and water solution. (Beers & Robert, 2002)
Propoxyphene
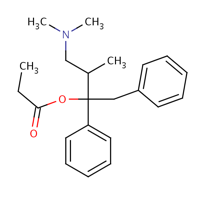
This drug works through the analgesic effect of the dextro-isomer and the antitussive effect of the levo-isomer. The molecular weight of this drug is 339.47; exists in solid form and has an experimental water solubility of 19.6 mg/l. This drug works through binding to the opiate receptors within the nervous structure leading to reduced pain stimuli perception. This drug is a weak agonist that works by acting on the OP receptors especially theOP3 that are united with the G-protein receptors to function as modulators.
By binding the opiate the exchange of GTP for GDP on the G protein complex is stimulated and further there is production of insulin; vasopressin and glucagons. This results to the closure of OP2 receptor agonists and opening of OP3 and OP1 agonists leading to overall hyperpolarization that suppresses neuronal excitability. The negative effects of this drug include respiratory depression; pulmonary edema and circulatory collapse among others. The half-life of reaction for this drug is 6-12 hours and is usually found in solid form. (Campbell, 2003)
On the area of conjugate acid-base pairing; Propoxyphene being a weak acid reacts to donate an H+ ion to form a conjugate solution. Propoxyphene hydrolysis is done using an acidic solution leading to the formation of a metabolically usable compound. Propoxyphene ionization takes place through the donation of an H+ ion to bond with the conjugating functional group leading to neutralization that forms salt and water. (Beers & Robert, 2002)
Naloxone
The chemical formula for the molecule of this drug is shown in the diagram:
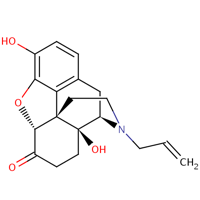
This drug works through affecting the mu; kappa and delta opioid receptors; has a molecular weight of 327.37 g/mol and exists in solid form with solubility 5.64e. This drug is used for the complete or partial reversal of the negative effects of natural and synthetic narcotics; these may take the form of respiratory depressions; depression and hypotension.
On the area of action; Naloxone works through antagonizing the opioid effects through struggling for the same receptor zones mainly the mu-receptor. However research has lately found out that Naloxone has binding effects on the other receptors like gamma and kappa. On the question of absorption, Naloxone is best absorbed when administered through intramuscular injection. Naloxone undergoes hepatic biotransformation and has a half-life of reactivity ranging between 30-81 minutes. (Campbell, 2003)
On the area of conjugate acid-base pairing; Naloxone which is a weak base reacts through donation of an A- ion to for a conjugate solution. Naloxone hydrolysis through the reduction of opioid activity led to the formation of casein formula. The ionization of this drug takes place through the donation of the A + ion to the reacting functional group that brings about neutralization leading to the formation of water and a salt solution. (Beers & Robert, 2002)
Pentazocycine
This drug has the molecular chemical structure shown below.
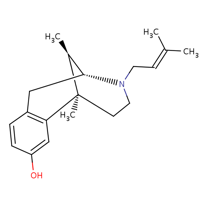
It’s the mixed agonist-antagonist pain reliever to be manufactured having an agonist function at the kappa and sigma opioid receptors; and a weak antagonist action at the mu-receptor. It has a molecular weight of 285.42 g/mol; exists in solid form and a water solubility of 1.22e. This drug is found to work significantly on administration after 15-30 minutes and is orally administered. The action duration is usually 3hours or longer and can be used to antagonize the analgesic effects of meperidine and morphine. It’s also found to be capable of partially reversing the behavioral depression; cardiovascular and respiratory difficulties induced by other Opioids like morphine. (Campbell, 2003)
Pentazocycine works through competing for receptor regions especially the mu receptor thus antagonizing the opioid effects of other drugs. This drug is best absorbed from the gastro-intestinal tract; undergoes hepatic biotransformation and has a half-life of reaction ranging from 2-3 hours. On the area of conjugate acid-base pairing; Pentazocycene being a weak acid reacts through donating an H+ ion for a conjugate solution. Pentazocycene hydrolysis is done using hydrochloric acid to make glycerinate; while the ionization of it takes place through donating the H+ ion to the conjugating agent leading to neutralization that results to the formation of a salt and water. (Beers & Robert, 2002)
Discussion
The explanation as to why effects of opioids like methadone effects last longer include the fact that its lipid solubility is quite high; the dependence incidence of patients is slow; and methadone metabolism is slow. This is the case due to the complex basic molecular composition of methadone and its ability to react within the organism for an extensive period of time ranging from 24 to 48. Another cause for this working of methadone is the genetic variability that is observed in the production of the associated enzymes. The fact that methadone is a soluble salt also makes the administration of this substance much easier as it is administered orally inform of a solution, and this helps improve the rate of intake as compared to other forms of administration like the tablet or the pill. This is the case because; through oral administration the drug goes directly into the gastro-intestinal tract that forms a major opioid reception region. (Campbell, 2003)
Most Opioids have the following molecular components: ditrideuteriomethylamino; dimethylamine hydrochloride; diphenylheptan one hydrochloride and as a result shows rather different physical and chemical properties when in different forms e.g. in solution, or solid. Methadone has basic properties; hydrophilic properties; and is depressive on metabolic processes. The fact that most opioids are acidic gives the idea that they most likely possess different ionization rates in different individuals depending on their genetic characteristics. These variations depend on the substances or drugs the individual had taken before the administration of the latter Opioid. (Campbell, 2003)
One of the adverse effects of Opioids especially Methadone is that it results to urinating difficulties and this can be used as a fact to justify the negative reactions of this drug in the human body that bring about dehydration. Methadone is often taken orally in the form of a solution; pill, or tablet and therefore this can be used to argue out that this drug has the property of being soluble in certain liquids. (Compton & Athanasos, 2003)
Alkylation is the exchange of an Alkyl group in the form of an akyl carbocation, carbine or carbanion from one molecule to the other. The Alkyl groups’ organic combination may take the form of methylation.The combination of one opioid with other opioids gives the justification that this process is involved in the chemical combining of the two compounds. Acylation is a chemical process through which an Acyl group is added to a compound. The compound that gives out the Acyl group is referred to as the Acylating agent and this process can be attributed to the compound combination of one opioid drug with other chemical compounds during medical applications. Phosphorylation is the adding up of a phosphate set to an organic molecule or a protein. This is a process used to turn many protein enzymes on and off that has the effect of stopping the development processes of diseases like diabetes and cancer. This chemical process maybe involved in the reaction of opioids depending on the donor properties of the reacting agents that are combining. (Compton & Athanasos, 2003)
Opioids being multiuse chemical compound have the capability for combining with other compounds on the basis of electrostatic attraction, and exchange as a result of the presence of charged groups within its compound form. Ionic bonding is the chemical bond that involves a metal and a nonmetal ion or polyatomic ions through electrostatic attraction of differently charged ions. (Kenneth, 2002)
The structural features of Opioids are varied as discussed previously. Opioids have the capability of making varied chemical combination when subjected to different reaction agents or physical processes. These variations in chemical structure include isomers that will have the same molecular formula but with different arrangement of the atoms forming the compound. The name given to the optical isomers of opioids is as a result of their respective effect on polarized light and these substances are referred to as enantiomers as they exist as two isomers. Diastereomers on the other hand occur, when two or more stereoisomers of a chemical compound have differences of structure at one or more ends therefore giving rise to two different configurations thus different stereoisomers. (Wilson, Caroline & Margaret 1999)
Talking of the relationship between the effects of molecular properties on the biopharmaceutical features of drugs can be explained using the common administration of drugs that is oral. This can be seen from the fact that oral bioavailability is one of the most essential considerations for the proper fashioning of bioactive molecules. As a result, poor oral bioavailability reduces drug working capabilities as it leads to high inter- and intra-patient variability.
As seen in the case of Methadone the high lipid solubility; the ability to dissolve and the slow metabolism rate affect the drug delivery of this drug, in that the lipid solubility enables it to be delivered to the different parts of the body and react with different target compounds. The slow metabolism rate that is also a molecular effect of the compound makes the reaction process more far-reaching and capable of suppressing pain for longer time in the case of analgesics. The high absorption rate affects the absorption levels of the drug and therefore has a direct effect on the transport and delivery to the different parts of the body. (Wilson, Caroline & Margaret 1999)
Drug receptors are molecules involved in the chemical transmission between and within cells and are found within the cytoplasm or the cell membrane. The level of interaction between the drug molecules and the receptors dictates the level of transmission of drugs between and within different cells. This has direct effect on the level of performance of a drug and is dictated by the Molecular composition and compatibility with the cells. (Kenneth, 2002)
The bio-transformation pathways of drugs is affected by; tissue localization of the metabolic processes; metabolic interactions; individual variability and polymorphism. However these factors affect the transmission pathways based on the chemical and molecular properties of the drugs, where those that are favorable to the process in terms of molecular composition are transmitted faster and better. (Compton & Athanasos, 2003)
The factors affecting drug metabolism include; age; pharmacogenetics; nutrition; sex; enterohepatic circulation and intestinal features. In this case metabolism is slower in elderly individuals than in ordinary adults among other factors. As a factor, enzyme induction or inhibition can also be attributed to different levels of drug metabolism as they either slow or increase the rate of metabolism.
In the drug metabolism enzyme system within the liver cell, injury occurs that is said to bring about hepatic metabolism but in this case its not considered. The strategies employed in managing drug metabolism include; reducing or increasing the level of intake of the drug; the use of a second messenger cell signal system like the hormone insulin that controls glucose levels; and injection of hormonal balancing strategies that help balance the levels of secretion of body fluids. (Campbell, 2003)
Pharmacological activity of body molecules is an explanation of the adverse or beneficial effects of drugs’ active ingredients that are however influenced by the other constituents of the drug. These pharmacological activities can show in terms of toxicity; stimulated body fluid formation and counteractive symptoms like allergies and dehydration. (Beers & Robert, 2002)
Conclusion
However it should understood that the use of Opioids as pain depressors or for other uses has both positive and negative outcomes. However the addiction; withdrawal syndrome or negative effects of one Opioid can be overcome through the use of a different one.
Reference list
Beers, M., & Robert, B. (2002). The Merck Manual of Diagnosis and Therapy. Whitehouse Station, NJ: Merck Research Laboratories..
Campbell, D. (2003) “Parenteral Opioids for Labor Analgesia.” Clinical Obstetrics and Gynecology 46, 616-622.
Compton, P., & Athanasos, P. (2003) “Chronic Pain, Substance Abuse and Addiction.” Nursing Clinics of North America 38, 525-537.
Kenneth, R. (2002).The Best Alternative Medicine, Part I: Western Herbal Medicine. New York: Simon and Schuster.
Wilson, B., Carolyn, L., & Margaret, T. (1999).Nurses Drug Guide 2000.Stamford, CT: Appleton and Lange.