Introduction
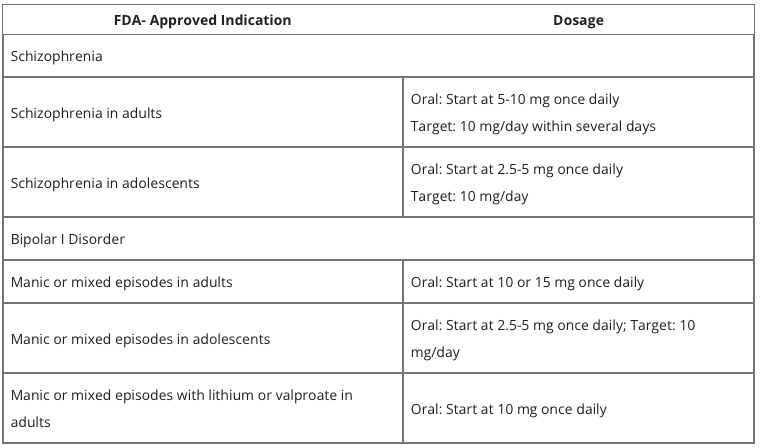
Olanzapine is an atypical antipsychotic principally prescribed to treat schizophrenia and bipolar disorder (it is also marketed under the brand name Zyprexa (Image 1)). FDA-approved indications related to the medication are presented in Table 1.
Non-FDA Uses
Olanzapine has the research supporting it for the off-label uses of dementia, anxiety, and OCD (Thomas & Saadabadi, 2022).
Classification
Olanzapine belongs to the group of drugs known as atypical antipsychotics. It functions by altering the way that a few organic brain chemicals behave.
The Medication Mechanism of Action
The drug mainly affects dopaminergic receptors in order to function. It functions as an inhibitor of the mesolimbic pathway’s d2 Receptors, preventing dopamine from potentially acting at the post-synaptic receptor.
The medication pharmacokinetics
Within the permitted dose spectrum, the pharmacokinetics of the medication is simple and dosimetric. In healthy people, it had a mean half-life of 33 hours and a range of 21 to 54 hours. The observed plasma clearance ranged from 12 to 47 L/h, with a mean of 26 L/h (Thomas & Saadabadi, 2022).
The Medication Pharmacodynamics
The medication is a second-generation antipsychotic that exhibits a variety of receptors’ sensitivities for neurotransmitter systems HTR2A and HTR2C, dopamine receptors (DRD1-DRD4), H1 Receptor, alpha1 transactivation, and neuromuscular junction receptors (CHRM1- CHRM5) (Thomas & Saadabadi, 2022).
Appropriate Dosing
Once daily, without regard to meals, the medication should be provided; the usual starting dose is 5 to 10 mg, with a goal dose of 10 mg/day in a few days. Given that a steady state for olanzapine would not be reached in the average patient for about one week, any more dose changes should typically take place at intervals of no less than one week. It is advised to increase or decrease the dosage by 5 mg QD as needed (Guzman, n.d.).
Considerations of Use and Dosing
In people who are elderly, have a history of hypotensive responses, are debilitated, display other characteristics that may impede the absorption of olanzapine, such as non-smoking women, or who are more pharmacodynamically responsive to olanzapine, the suggested beginning dose is 5 mg (Guzman, n.d.). The suggested beginning dose of oral olanzapine for teenagers is 2.5 or 5 mg, with a goal dose of 10 mg/day, and it should be given once daily without regard to meals. The drug is not recommended for children and during pregnancy.
Half-life
For any particular medication, understanding the idea of half-life is helpful in calculating steady-state levels and excretion rates. The half-life of olanzapine ranges from 21 to 54 hours, with a typical of 30 hours. The stable plasma levels of olanzapine are reached after roughly a week of daily treatment. Olanzapine thus exhibits linear pharmacokinetics within the FDA-approved dosing range (Thomas & Saadabadi, 2022).
Side Effects
Possible side effects include increased hunger, constipation, sore throat, dizziness, lightheadedness, stomach discomfort, and weight gain. The likelihood of falling might rise if one feels lightheaded or dizzy.
Contraindications
Patients who have a known sensitivity to olanzapine or drugs in its class should not use it. Olanzapine carries a black box warning for dementia-related psychosis. Due to a higher risk of death, olanzapine should not be administered to elderly dementia sufferers who also exhibit psychotic symptoms (Thomas & Saadabadi, 2022). Additionally, doctors should prescribe this drug cautiously in patients who are obese or even have diabetes owing to the negative consequences of gaining weight and metabolic disorders.
Overdose
CNS depression with drowsiness, impaired vision, hypotension, respiratory depression, neuroanatomical and antispasmodic effects, as well as unusually high temperature are the most typical symptoms that come from olanzapine overdose.
Diagnostics and labs monitoring
The concentration of olanzapine in the blood is dose-dependent, but many other factors can have a significant impact on its level in the blood. For this reason, when prescribing olanzapine, it is advisable to conduct drug monitoring. Diagnostics and lab monitoring of olanzapine are of particular importance in the absence of response to therapy with this drug. In such a situation, a low concentration of the drug in the blood may indicate non-compliance of the patient with the drug regimen. Drug monitoring allows one to timely adjust the dose of the drug and prevent the occurrence of side effects. This is of particular importance, given that the development of side effects often leads to the patient’s reluctance to follow the drug regimen and self-withdrawal of the drug.
Comorbidities Considerations
Olanzapine has cardiometabolic side effects and is linked to weight gain. Weight gain brought on by antipsychotics is connected to treatment pauses, thus raising the likelihood of recurrence and hospitalization (Meftah et al., 2020).
Legal and Ethical Considerations
No legal considerations are associated with the medication – except the apparent fact that this drug can be sold only with the necessary prescription from the physician. From an ethical perspective, it is debatable whether antipsychotics should be used to treat dementia’s behavioral and psychological symptoms. Antipsychotics have negative effects, and evidence-based recommendations discourage their usage. It is argued that the palliative paradigm may be used to justify the use of antipsychotics by lowering severe suffering in people with short life expectancies.
Patient Education
Olanzapine should be taken every day at about the same time. One should ask their physician or pharmacist to clarify any instructions on the prescription label that one is unsure about. The patients should never take it in larger or fewer amounts or more frequently than directed by the doctor. Moreover, it is essential for patients to monitor their weight, given that the drug is associated with weight gain (Citrome et al., 2019). The latter indicates that the organism cannot manage the medication appropriately, and alternative treatment or drug is needed.
References
Citrome, L., McEvoy, J. P., Todtenkopf, M. S., McDonnell, D., & Weiden, P. J. (2019). A commentary on the efficacy of olanzapine for the treatment of schizophrenia: The past, present, and future. Neuropsychiatric Disease and Treatment, 15(1), 2559–2569.
Guzman, F. (n.d.). Olanzapine indications: FDA-approved-uses. Psychopharmacology Institute.
Meftah, A. M., Deckler, E., Citrome, L., & Kantrowitz, J. T. (2020). New discoveries for an old drug: a review of recent olanzapine research. Postgraduate medicine, 132(1), 80–90.
Thomas, K., & Saadabadi, A. (2022).Olanzapine. StatPearls.
Wellona Pharma. (n.d.). Olanzapine Tablets.