Background
The ozone layer is a blanket of naturally occurring gas that is located in the stratosphere (15 to 50 km above earth) and serves a very important role in blocking the sun’s harmful ultraviolet-B rays from reaching us on earth. However, this protective layer of gases has been undergoing a slow but steady reduction in size. Ozone depletion has been taking place since the 1960s and is now a global phenomenon that requires urgent attention.
Ozone (O3) is a gaseous compound made up of three oxygen atoms and is continually being formed and decomposed in the stratospheric zone. The major cause of this depletion is the continued use of substances containing chlorine and bromine, known as chlorofluorocarbons (CFCs).
Once the CFCs reach the stratosphere, they are broken down into constituent elements and react with the highly reactive ozone molecules, thereby reducing the size of the ozone layer. The U.S. Environmental Protection Agency estimates that a single chlorine atom can decompose more than 100,000 ozone molecules (Newman et al).
While the largest ozone depletion is recorded at the southern and northern hemispheres, the process is taking place everywhere but is minimum in the tropics. This problem has led to increased levels of exposure to UV-B radiation that will continue to have adverse effects on all people living on earth irrespective of their location or economic status.
Individuals with lightly colored skins are more vulnerable to the cancerous effects of UV-B radiation. UV-B radiation also has an impact on plant life, which in turn creates an imbalance in the ecosystem. Scientists have mentioned that depletion of the ozone layer will also reduce fish stocks in lakes, rivers, and seas hence creating a food shortage in areas that heavily depend on fish.
Ozone Cycle
Formation of ozone begins when oxygen molecules decompose after absorbing UV light with a wavelength that is less than 240nm in the stratosphere, producing two oxygen atoms. One oxygen atom then reacts with an oxygen molecule to give ozone. The ozone molecule absorbs ultraviolet radiation of wavelength 310 and 200 nm, this decomposes it to oxygen molecule and an oxygen atom.
The oxygen atom then reacts with an oxygen molecule to form ozone. The process is continuous and is known as the ozone-oxygen cycle. The cycle stops when an oxygen atom reacts with ozone molecule to give two oxygen molecules, i.e.
Oxygen-Ozone Cycle
O2 -UV radiation (<240 nm)-> 20
O + O2 -> O3
O3 -UV radiation (310-200 nm)->O + O2
Process termination
O + O3 ->2O2
Generally, the level of ozone in the stratosphere is controlled by the balance between the photochemical production of oxygen atoms and the recombination reaction.
Destruction of the ozone occurs when free radicals reach the stratosphere, these radicals include the hydroxyl radical, nitric oxide radical, chlorine radical and bromine radical. Hydroxyl and nitric acid radicals reach the atmosphere through natural ways, however, chlorine and bromine radicals are due to man’s activities and are found in certain stable compounds, especially CFCs (McFarland, pp. 1207).
CFCs can reach the atmosphere without decomposing into their constituent elements since they are stable and non-reactive. When CFCs reach the stratosphere, they undergo photochemical decomposition to release chlorine or bromine atom, i.e.
CFCl3 -> CFCl2 + Cl
The liberated chlorine and/ or bromine atoms destroy ozone molecules through a series of catalytic reactions (Solomon et al, pp. 412). In an elementary example of this reaction, a chlorine atom reacts with ozone molecule forming chlorine monoxide, ClO, i.e.
Cl + O3 -> ClO + O2
The chlorine monoxide is unstable and can readily react with another ozone molecule to give two oxygen molecules as shown below:
ClO + O3 -> Cl + 2O2
This reaction reduces the number of ozone molecules in the stratosohere.
A single chlorine atom would continuously destroy ozone molecules for up to two years, however, other reactions in the stratosphere remove these elements. Bromine is more destructive than chlorine. Both of these elements are present in the stratosphere and cause considerable damage to the ozone layer. A single chlorine atom is able to destroy nearly 100,000 ozone molecules, when we consider the amount of CFCs released into the atmosphere annually, the damage done to the ozone layer becomes apparent (Storlaski et al, pp. 1015).
Trends in Ozone Layer Depletion
Studies of the ozone layer have been undertaken since the mid 20 the century, however, changes in its size became apparent between 1960 and 1970 when it was observed that it had reduced by 23 per cent between this duration. In 1985, the ozone hole was first observed in the Antarctic. By 1986, three models had been postulated to explain ozone depletion:
- Solar cycle model- regular increases in the quantity of nitrogen oxides in the lower Antarctic stratosphere is due to changes in solar radiation;
- Dynamical model- an alteration in the circulation pattern from downwelling of air with a high abundance of ozone from the upper stratosphere to upwelling of air deficient of ozone from the troposphere; and
- Halogen model- a number of variant theories centering on the catalytic destruction of ozone layer because of CFCs and halons (Storlaski et al, pp. 1015).
A study was undertaken by McMurdo in 1986 that showed that the concentrations of nitrogen oxides were remarkably low, hence disapproved the solar cycle model. He also observed that the levels of long-lived tracers were due to complex reactions and was unlikely to occur, and that the high levels of chlorine in the stratosphere was the most probable cause of ozone depletion (Storlaski et al, pp. 1015).
The Montreal Protocol was signed in 1987, although the origin of the ozone hole was not well understood, the protocol recognized that the stratosphere had been disturbed. It was observed that the levels of chlorine in the atmosphere had increased by about 5% and that there had been a considerable loss in ozone every October in the Antarctica (Newman et al). The Montreal Protocol was signed by 31 countries and was aimed at cutting CFC emissions as shown below:
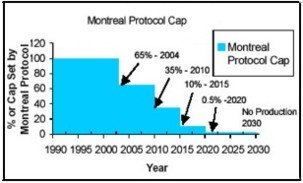
Fig. 1: Montreal Protocol Cap
The pact has been ratified by 197 nations to date and aims at reducing CFC emissions gradually so that no emissions will be made by 2030.Unfortunately, only a few countries have implemented the emission control mechanisms.
Advances in scientific methods improved man’s understanding of the ozone layer from 1987 -92. This enabled scientists to re-assess present and early trends in the stratospheric ozone.
Studies undertaken in Punta Arenas, Chile, in 1987 that involved making flights above the Antarctica gave a good understanding of the role of CFCs in ozone depletion (Farman et al, pp. 209). Chlorine and low concentrations of bromine were detected in this area, the study also found out that the level ClO was considerably high.
Other observations such as the presence of bromide radical (BrO), low concentrations of water vapor, nitrogen oxides, and nitrous oxide, and polar stratospheric clouds confirmed that the ozone hole was formed due to the catalytic destruction of ozone by chlorine radicals (Weatherhead & Andersen, pp. 40).
Effects of Ozone Depletion on the Environment
Ozone depletion affects man and the fauna and flora on the earth’s surface. The most common form of skin cancer in man, basal and squamous cell carcinomas, have been attributed to exposure to ultraviolet-B (UVB) (Kelfkens et al, pp. 821). Absorption of UVB radiation causes faults when DNA replicates, causing cancer.
Studies have shown that a 1% decrease in the ozone would increase the frequency of this form of cancer by 2%. Another form of skin cancer, malignant melanoma, is caused by exposure to ultraviolet-A radiation, however, some studies have shown this cancer is caused by exposure to UVB radiation.
A relationship also exists between cortical cataracts and exposure to UV-B radiation. Scientists have also said that a rise in ozone depletion may increase the occurrence of malaria and other infectious diseases while the EPA forecasts that close to 60 million Americans born by 2075 will have skin cancer (Zehr, pp. 608).
A November 2010 research at the Institute of Zoology in London revealed that whales off the coast of California coast exhibited a drastic rise in sun damage, they attributed this observation to the depletion of the ozone. The study identified “widespread evidence of epidermal damage commonly associated with acute and severe sunburn,” having cells that are formed when DNA is damaged by UV radiation.
These findings suggest “rising UV levels as a result of ozone depletion are to blame for the observed skin damage, in the same way that human skin cancer rates have been on the increase in recent decades.” Besides, skin cancer associated with man due to exposure to UVB has also been identified in animals (Zehr, pp. 607).
Increase of exposure to radiation affects crops and other plants. Economically important crops such as rice and beans depend on cyanobacteria living on their roots to help in nitrogen fixation, however, cyanobacteria are responsive to UV light and would be easily affected by increase of exposure to radiation.
Oceans will also be affected: microscopic organisms such as planktons may not survive in the future, this would imply that animals above planktons in the food chain would not survive. Fish numbers will dwindle and this will in turn. Changes in climate could occur and this would have damaging effects on earth’s balance.
Possible Solutions to Ozone Depletion
Due to the grave effects of ozone depletion, we must strive to work towards abating and reversing the damages that have been done to the ozone layer. Some of these solutions are outlined below:
- Since ozone depletion is mainly caused by CFCs, the first solution would be to ban the use substances containing the se substances. However, this would have to be followed with tough legislations to punish offenders. The tough regulations would compel countries to observe minimum CFC emission into the atmosphere (Norman et al, pp. 331).
- Scientists have come up with a plan to introduce chemicals into the stratosphere to prevent the formation of ozone depleting substances. This plan would involve depositing about 50,000 tons of hydrocarbons (CH3 or CH4) into the atmosphere, the following reaction is expected:
CH4 -> CH3 + H
Cl + H -> HCl
The challenge with this method is that ethane or propane would decompose after one year and the expensive would have to be repeated annually(Norman et al, pp. 332).
- A solution to this problem lies in adopting simple strategies that can be practiced by anyone. These include recycling CFC containing substances such as plastics to reduce their production.
Conclusion
Depletion of stratospheric ozone layer by chlorofluorocarbons is a universal problem. While the issue was a matter of scientific guesswork in the mid 20th century, it is now an urgent policy question for governments around the world. The weight of scientific evidence strongly points to CFCs as the main source of the reduction in stratospheric ozone over the Antarctica.
Satellite images supported by ground-based surveillance show that ozone has been declining since the late 1960s in both hemispheres, and this decline cannot be explained by any known natural processes. In weight of this evidence, we can conclude that halogenated compounds are largely responsible for the depletion of the ozone.
Scientists have mentioned that the ozone hole will be around for the next 50 years, and its repair can only start in 2020. However, this does not bar us from carrying out activities aimed at reducing and reversing depletion of the ozone layer.
More than two decades after the Montreal Protocol was signed, the world is yet to get tangible results from these efforts. This stems partly from the lack of stringent legislation to aid in implementation of the agreement and can be compared to the Kyoto Protocol.
A working solution to lessen the depletion of the ozone would be to limit the quantity of CFCs released in the atmosphere. All countries would sign a deal that binds them to the pact. To ensure that all countries adhere to the pact, meetings would be held after every three years to check on the progress of its implementation.
This would also be a time to evaluate the challenges that each country faces, and find possible solutions. This solution stems from the failure of the Montreal Protocol to reduce the emission of CFCs into the atmosphere. Adequate protection of the ozone layer will require the full co-operation between all countries, both developed and developing.
Works Cited
Farman, Joseph C., Gardiner, Brian G., and Shanklin, Jonathan D. Large losses of total ozone in Antarctica reveal seasonal CLOx/NOx interaction, Nature, 315, 1985. 207-10.
Kelfkens, Gert, de Gruijl, Frank R., and van der Leun, Jan C. Ozone Depletion and Increase in Annual Carcinogenic Ultraviolet Dose. Photochem. Photobiol. 52, 1991. 819-823.
McFarland, Mack. Chlorofluorocarbons and ozone, Environ. Sci. & Technol., Vol. 23, No. 0, 1989. 1203-08.
Newman, Paul. A., Kawa, Randolf and Nash, Erick R. (2004). On the size of the Antarctic ozone hole? Geophysical Research Letters 31: L12814.
Newman, Paul. A., Nash, Erick, Kawa, Randolf, Montzka, Steve A. and Schauffler, Sue M. When will the Antarctic ozone hole recover? Geophysical Research Letters 33: L12814, 2006.
Norman, Catherine, DeCanio, Stephen, Fan, Lin. The Montreal Protocol at 20: Ongoing opportunities for integration with climate protection. Global Environmental Change 18 (2): 2008. 330–340.
Solomon, Phillip, Connor, Brian, de Zafra, Robert, Parrish, Allan, Barrett, James, and Jaramillo, Matt. High concentrations of chlorine monoxide at low altitudes in the Antarctic spring stratosphere: secular variation. Nature 328 (6129): 1987. 411–3.
Stolarski, Richard, Bloomfield, Peter, McPeters, Rich, and Herman, Jack. Total ozone trends deduced from Nimbus-7 TOMS data, Geophysical Research Letters, 18, 1991. 1015-18.
Weatherhead, Elizabeth, and Andersen, Signe Bech. The search for signs of recovery of the ozone layer. Nature 441 (7089): 2006. 39–45.
Zehr, Stephen C. Accounting for the Ozone Hole: Scientific Representations of an Anomaly and Prior Incorrect Claims in Public Settings. The Sociological Quarterly 35 (4): 1994. 603–19.