Introduction
Recently, the pharmaceutical industry has grappled with an increased cost in production partly because of uncertainty with regards to the necessities for regulatory compliance. Of particular interest is the validation of particularly the automation systems as well as the accreditation of Practices for Heating, Ventilation and Air Conditioning (HVAC).
Initially, the existence of many yet acceptable interpretations of these regulatory requirements led to confusion between manufacturers leading to inconsistencies in processing practices. With the inception of these practices, a part from increased costs in production, there has been a decrease in the rate at which new products come to market. (World Health Organization, 1997)
In the year 1994, a body representing the pharmaceutical engineers in conjunction with both the International Society for Pharmaceutical Engineers (ISPE) and the Food and Drug Administration (FDA) agreed on a common course that led to the creation of Baseline Pharmaceutical Engineering Guides.
These guides are aimed at aiding pharmaceutical manufacturers “in the design, construction and commissioning of facilities that comply with the requirements of FDA” (ISPE Baseline Pharmaceutical Engineering Guides for New and Renovated Facilities, 1999).
As such, pharmaceutical industries are required to meet the current good manufacturing practices (cGMPs) which ought to coincide with the entire governing laws and policies. The joint interpretation of these regulations is important for the purpose of consistency, flexibility and enhancement of innovative approach in the design, construction and validation.
The scope of this guideline is limited to the development of new products as well as the existing ones which tend to have limited baseline description. However, these guidelines are not intended to substitute the existing laws and regulations which apply to the same. To supplement this document, there is need to incorporate the existing laws and regulations to the same for the purpose of completeness.
Basically, this guide owes its guidance from the following sensitive parameters: the critical processing step, product exposure, level of protection, critical parameters, critical instruments and systems, Good Engineering Practice (GEP) and enhanced documentation.
With regards to critical processing step, this is significant in defining consistent regulatory requirements and as such, it specifies the extent of product exposure and the level of protection.
However, due to environmental regulations upon a specific methodology employed, standard operating procedures (SOPs) are used hence; this enhances flexibility in manufacturing designs thereby reducing the processing costs within the regulatory requirements.
With regards to critical parameters, this has an effect on the quality of a product. This ought to be identified, regularly checked and controlled to maintain the product quality. In order to identify these parameters, manufacturers should have knowledge about the processing steps and as such document the rational for afterward examination.
Critical parameters define critical instruments and systems and just like the parameters, they require in-depth documentation. As regards GEP’s, the guide requires that all processing elements in a facility to “routinely undergo some form of commissioning” (Milton, 2002).
Basically all the engineering aspects of a processing system needs to be inspected regularly, tested and above all recorded down for documentation. GEP requires that prior to setting of the plant, all the stakeholders be involved in “the planning, design, construction and commissioning phases to ensure systems are documented once” (Latham, 1995). Enhanced documentation is a plus to the Good Engineering Practices.
The essence of doing an exhaustive documentation stems from the fact that most systems and commissioning documents do not undergo regular update long after inception. Regulations entail change control with respect to certain document. Moreover, validation for the regular inspection of the critical systems to enhance consistency in quality has to be supported by documentation (Wichmann, 1997).
Guide with respect to design of sterile manufacturing facilities
Traditionally, the design and construction of a Bulk pharmaceutical chemical (BPC) plant is just like a chemical manufacturing plant and as such, they have ceased from being pharmaceutical dosage-form industries.
While chemical manufacturers give tolerances for traces of contaminants in the final product, “pharmaceutical facility and processing design requires provision for minimizing cross contamination and trace contamination” (International Conference on Harmonisation (ICH), 2009). In the ISPE guide concerning the recent facility design of BPC, the principles are based on the dosage-form pharmaceutical industries.
Consequently, the guideline has become a vital tool in helping a project team meet the minimum restrictions for a facility design in line with cGMPs requirements. Just like in BPC, ISPE gives guidelines with respect to sterile chemicals manufacture in ‘Sterile Manufacturing Guide’. This document was obtained courtesy of great minds in the pharmaceutical fraternity composed of a task force of 50 personalities.
The essence of this guide which dwells on engineering issues is meant at providing cost efficient facilities. It generally focuses on the aseptic processes that ultimately lead to terminal sterility of the final product.
The primary features of this guide are: Product requirement, “GMP critical parameters” and “Critical Devices”, Terminal sterilization, Aseptic processing area, Protection of the product, Flow of people and material, integrated facility design, Barrier-isolator technology, Consistent HVAC principles, In operation condition for HVAC, Good Engineering Practice, Direct impact systems, Enhanced documentation and Indirect impact systems (International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH), 2007).
The product requirement decides the vital aseptic needs of a given facility and as such, the ‘critical parameters’ can be established. The aseptic processing area is a region where formulation of the product takes place after which is packaged and sealed. This is a critical area where control of persons and materials ought to be perfect to bar cross contamination.
An efficient way of a voiding this is by barrier-isolator technology which ought to be incorporated in the design initially during installation. HVAC principles give the baselines for aseptic manufacturing processes. Engineers and designers should take heed of this stage of operation where “regulators are particularly interested with the in the environment during in-operation condition” (Orange Guide, 2007).
This is so because it is believed that this is the time when the product may be exposed. Designers should be in a position to identify the potential sources of microbial/ particulate contamination and the ways of ensuring quality air free of contamination.
Baseline standards also come in handy in the selection of materials as well as finishes since this impact directly on the quality of the final product. It would be insignificant for one to spend much in instrumentation and control yet the no GMP is achieved. As regards Good Engineering Practice, this needs to be applied to the entire facility to ensure compliance of the product with respect to quality needs.
This guide brings forth the term ‘direct Impact System’ which basically means the facilities which have a direct impact on the product. Moreover, it highlights the term ‘Indirect Impact System’ which generally means the opposite of the former term. These systems ought to be “supported by enhanced documentation” (International Cleanroom Standards, 2007).
Qualification for compressed air
The facilities designed to support pharmaceutical operations ought to comply with the GEP and cGMP. These systems just like as it has been stated initially, they may have either direct or indirect impact on the quality of the product.
The former system ought to be comprehensively documented and inspected with respect to critical GMPs limits. The stakeholders should agree on the degree of qualifications prior to the installation process. The impact assessment process is represented in the flowchart below:
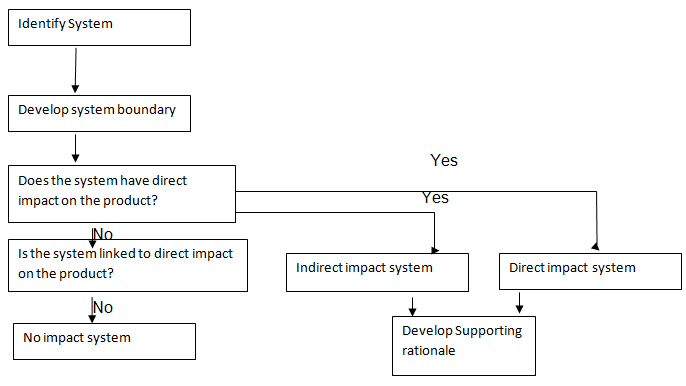
With regards to direct system, the fundamental parameters that ought to be analyzed are: purified water, water for injection, clean steam and HVAC and compressed special gas. The indirect systems that need to be checked are raw water treatment, cooling system, effluent treatment, heating system and boiler house.
Commissioning overview normally takes “equipments from installation to operation as well as incorporating a systematic method for testing and documentation” (European Commission, 2005). Both commissioning and validation procedures come up with equipment lists, component lists, utility verification forms, systematic drawings and operating procedures.
However, while validation focuses on user responsibility, commissioning focuses on supplier responsibility. Moreover, while validation is approved by the quality assurance team, commissioning is approved by engineering project team. Since compressed air system is a direct system, qualification work is needed.
User Requirement Specification (URS) requires that quality of air be generated from the system at generation and point of use is determined. Furthermore, it calls for safety measure and prevention of contamination.
Qualification for Nitrogen gas
Air monitoring methods are used to regulate the emission of dangerous gases in the environment by keeping them within the set emission limits. The gases with limited emission limit include Carbon monoxide, compounds of Nitrogen Oxides, Ozone, Sulphur Dioxide and Hazardous air pollutants. The roles of industries are to mitigate these toxic emissions within safe limits.
This is achievable through “Air Pollution Control Devices that include: Mechanical collectors (Hepa filters), Hazardous solvents (thermal oxidation, gas absorption scrubbers and adsorption) and selective catalytic reduction techniques” (Daly, 1985).
Qualification for Steam systems
Steam is widely used in processing of pharmaceutical products important for treatment. Steam exhaust from boilers also referred to as utility steam come in contact with products directly acting as potential source of ‘direct impact system’.
This may be in form of condensate which settles on the products depositing contaminants (rust and additives) on the product. The quality of steam is determined by the Good Manufacturing Practices (GMPs) which determines the final quality of the product. Steam comes in handy when carrying sterilization processes that include:
Manufacture of Injectable or Parenteral solutions, which are always sterile. Biopharmaceutical manufacturing and manufacture of sterile solution e.g. ophthalmic products (D’Elia 1994).
Clean steam may cause contamination through humidification among other forms of contamination. Clean steam system design enhances formation of quality products. This is achieved by: “avoidance of corrosion, prevention of entry of contaminants into the system and, preventing microbial growth in the system” (Reeuwijk, 1998).
For the purpose of validation process of steam utility, a sequential process ensures generation clean steam: “Develop a User Requirement Specification (URS), develop a Functional Specification (FS), Undergo Design Qualification (DQ), Installer Qualification (IQ), Operational Qualification (OQ) and finally Performance Qualification (PfQ)” (Commission Directive 2003 EC, 2003).
Conclusion
In a conclusion, for any pharmaceutical manufacturing industry, the need for ISPE Guide in the initial installation of facilities is vital to minimize cost due to redesigning of the entire system. The validation and commissioning of the processes should be done for once and for all by involving all the stakeholders to limit redesigning costs.
Essentially, by implementing ISPE guidelines one will have basically met all the requirements for accreditation procedures set for pharmaceutical industry. This is so because it coincides with the requirements for FDA and WHO (Heinemann, 2003)
Bibliography
Commission Directive 2003 EC, 2003. Laying dawn the principles and guidelines of good manufacturing practice in respect of medicinal products for human use and investigational medicinal products for human use. London: Department of Health.
D’Elia, L., 1994. “Bioprocess Engineering-Systems, Equipments & Facilities”.Utility for Biotechnology Production Plants. New York City: John Wiley and Sons, Inc.
Daly B., 1985. Woods practical guide to fan engineering. Colchester, Woods of Colchester Ltd. Third impression. Cambridge: Cambridge University Press.
European Commission, 2005. The rules governing medicinal products in the European Community, Volume IV. Good manufacturing practice for medicinal products. European Commission. Brussels: ViVio. Retrieved from<https://www.cen.eu/Pages/default.aspx>
Heinemann, D., 2003. Good Laboratory and Clinical Practices, Techniques for the Quality Assurance Newnnes, Oxford.
International Cleanroom Standards, 2007. International Organization for Standardization. Brussels: ViVio. Available at: <https://www.iso.org/developing-standards.html>
International Conference on Harmonisation (ICH), 2009. Quality Risk Management – 09. London: Department of Health. Web.
International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH), 2007. Good Manufacturing Practice Guide for Active Pharmaceutical Ingredients – 07 , London: Department of Health. Retrieved from: ISPE Baseline Pharmaceutical Engineering Guides for New and Renovated Facilities, 1999. Sterile Manufacturing Facilities, First Edition. London: Department of Health.
Latham, T., 1995. “Clean Steam Systems”. Pharmaceutical Engineering.15 (2), p. 3.
Milton, A., 2002. GLP Quality Audit Manual. Interpharm Press, third edition, ISBN 1-57491-106-6 (2002).
Orange Guide, 2007.‘Rules and Guidance for Pharmaceutical Manufacturers and Distributors,” commonly known as the,’ MHRA, February 2007, New York University Press.
Reeuwijk, P., 1998. FAO Soils Bulletin 74, Guidelines for quality management in soil and plant laboratories, New York University Press.
Wichmann, B., 1997. Software in scientific computing, National Physical Laboratory. Measurement Good Practice Guide No. 5. Cambridge: Cambridge University Press.
World Health Organization, 1997. Quality Assurance of Pharmaceuticals. A compendium of guidelines and related materials, Volume 1. Geneva: ILO Publications.