Abstract
A flow cytometry method for enumerating levels of anti-D is mainly based on the indirect antiglobulin assay. The technique uses FITC Fab anti-human reagent for recognition of bound of anti-D. A fluorescence is measured by flow cytometry by means of a typical anti-D preparation which is used to engender a standard curve. By the use of the produced standard curve, the fluorescence attained from the analyzed model by flow cytometry is changed into IU/Ml anti-D (1). There are three available flow cytometric techniques that are aimed at anti-D quantification. Each and every one of these methods uses diverse cell sensitization, as well as different antibody recognition circumstances. The variation in antibody detection and also in cell sensitization of the various techniques may lead to wide range of outcomes and this call for a timely direct contrast of the above techniques.
The quantification of anti-D by flow cytometry is usually influenced by the serum antibody characteristics, as well as the technique employed (2). Flow cytometry is an undemanding method to carry out, and thereby has the potential to put back the AutoAnalyser. Differences in the two techniques is that AutoAnalyser and flow cytometry point out that additional validation of the flow cytometric process is necessary prior to routine application. The flow cytometry process for quantification of anti-D employs the principle of light excitation, light scattering, as well as fluorochrome molecules for data production from cells and particles. In regard to dependability, as well as sensitivity, flow cytometry recommends an extensively used method with an appropriate property.
Introduction
Flow cytometry is a technique which is used to diagnose various diseases, especially the ones related to the blood cancers. This technique is widely used nowadays in order provide chemical and physical analysis of the particles and detect possible disorders. This technique is used to count microscopic particles. Thus, the particles are suspended in the flow of liquid and detected with the help of electronic apparatus. These days, flow cytometry is widely used in various clinic laboratories in order to monitor blood related diseases. For example, this universally adapted technology was used to analyze T-lymphocyte which “functional assays were needed in cultures of human blood in order to detect the potential and the heterogeneity of subpopulations” (3). These days, this technique is also used to count the Rho(D) immune globulin, or simply anti-D which is used to prevent “Rhesus deseases”.
The quantification of anti-D is normally carried out by the use of a continuous flow system where sensitized cells are agglutinated and done away with, as the remaining ones are lysed (4). Standard preparations of anti-D are used to establish antibody concentration that is inversely proportion to hemoglobin absorbance. An AutoAnalyser is another technique that is broadly employed despite the fact that it has a plenty of intrinsic complexities that might have consequences in huge inter-assay, as well as intra-assay disparities. Due to the difficulties that came up considering the use of an AutoAnalyser, an option of using a flow cytometry was established wherein the anti-D bound to the plane of the red cell was perceived by means of a Fab fragment of an anti-human complexity IgG that is fluorescently marked. From a standard IgG preparations, a curve is a product that is used to determine the quantity of anti-D in the serum test (5). The quantification of anti-D antibodies in sera from an vaccinated women is a crucial diagnostic practice process to foresee the hazard of hemolytic disease of the infants. The flow cytometry technique provides a broadly utilized process with the appropriate properties with regard to dependability, as well as the sensitivity. Prior to using the flow cytometry as a typical process, various methodological facets are supposed to be considered.
In the anti-D quantification, flow cytometry is utilized. This technique uses the law of hydrodynamic focusing. When the particle of concern interrupts the origin of light, they disperse light hence leading to flourochrome being thrilled to elevated vigor states (6). The vigor is then unconfined as a light photon which has explicit spectral characteristics. Quotation of anti-D by flow cytometry is getting increased in use in routine laboratories. This, however, has lead to the increase in the disease detection, as well as prognosis, thus leading to development of improved ways of detecting, as well as predicting the diseases. In the quantification of anti-D, the sensitized red cells are initially agglutinated after which they are removed and the remaining cells are lysed (7). Standard preparations of anti-D are used to determine antibody concentration that is inversely proportional to hemoglobin absorbance. Even though, the autoanalyzer method is broadly utilized, it has plentiful of inherent complexities. These difficulties may consequence to greater interassay, as well as intraassay disparities. Both the autoanalyzer and the flow cytometry methods, peruse the fundamental format described, thus giving a good association with the anti-D values that had been attained by the auto analyzer. On the other hand, a number of serum samples offer dissimilar anti-D concentrations by the flow cytometry and also the autoanalyzer techniques time after time.
In a number of studies, a comparison of all the method was used to find out if the process manipulates the degree of divergence of the selected models from the autoanalyzer out comes (8). The presence of either anti-E or anti-C component generated high values that made them to be substituted with R2R2 so as to keep them out for a quality standard curve. With a number of serum samples, the prevalence of diluents for cells and the serum influence the value of anti-D acquired. The disparities in antibody composition, as well as the heterogeneous assortment of affinities viewed in serum samples, may be reflected by the fact that the selection of diluents influences the value acquired. The utilization of low ionic salt solution all through the flow cytometric test notably boosts the observed concentration of anti-D, although the resulting value do not compare that of the autoanalyzer. When low ionic salt solution is employed as a diluents instead of PBS, the uptake of anti-D is superior and, at the same time, this disparity can be further improved with BAS/ low ionic salt solution. As a result, the antibodies that are normally uncreative at low concentration are detectable with low ionic salt solution. Serum-to-packed-cell ratio has an effect on the quantity of antibody bound per cell.
The major differences between the flow cytometry and the autoanalyzer methods point out that more justification of the flow cytometry is needed prior to the usual exploitation (9). Both techniques for quantification of anti-D, that is the flow cytometry, and the AutoAnalyser pursue the fundamental design. Although their similarities the two techniques have some differences which include:
- The cell concentration
- The cell phenotype an
- The diluents
In spite of these variations, all the techniques are portrayed as giving an excellence link with the anti-D values that are acquired by the Auto Analyser. On the other hand, it has been observed that a certain number of serum samples, over and over again, present dissimilar anti-D concentrations by the flow cytometry, as well as by the Auto Analyser techniques. Sera obtained from a vaccinated female for the quantification of anti-D is an essential investigative practice method. This test is crucial due to the fact that it helps scientists in forecasting the danger of the hemolytic illness of the infant. Flow cytometry is a method for analyzing, as well as reckoning minute units like the chromosomes and also cells (10). This process involves dangling these microscopic units in a flow of fluid, and passes them via electronic detection equipment. This process allows for concurrent multiparametric scrutiny of the chemical, as well as the physical features of numerous atoms in a short duration. There is a vast number of applications, as well as uses of a flow cytometry which engrosses:
- For analysis of health disorders like blood tumors
- For clinical practices
- Research purposes
- Prediction of illnesses
- Monitoring of disease.
Materials and methods
Donor red cells
Three O blood groups Rh (D)-Positive with diverse phenotypes were collected in a ACD tubes and kept at temperatures of four degrees. Some of the ACD blood was transferred into a glass tube. Two of the O blood groups used were R1R1, while the third one was R1r. As well, R2R2 was also employed with the test of quantifying unidentified serum samples. The transferred blood was poised in 1% phosphate buffered saline and automatically washed three times. A supernatant layer that was formed was discarded and the red cells obtained from three different individuals (11). One of the individual’s blood cells was used in the succeeding tests, as no varying outcomes were observed.
Serum samples
Six frozen sera samples containing D antibody obtained from immunized women. They were used for a variety of tests. A few of these samples contained either anti-E or anti-C or even a combination or both.
Antibodies
AHG IgG-FITC was used in the initial standard curve experiment. FAB fragment that was fluorescently coagulated of affinity purified anti-human IgG that was used as a secondary antibody.
Anti-D standard
27.7 l U/ml of standard concentration from CSL were utilized to prepare the standard curve. Different concentrations of the standard concentration (0, 0.125, 0.25, 0.5,1,2,4,6,8,10 Lu per ml) were diluted in LISS/5%BSA
Flow Cytometer
A flow cytometry BD FACS canto was used for acquisition, as well as for the data analysis. The selected flow cytometry for use had a wavelength of 488 nm and FITC conduit with green light.
Statistics
y=a+bx is the formula that was applied in the calculation of the linear regression. The correspondent coefficient that is (r) was calculated for each standard curve. For the comparison of autoanalyzed with the flow cytometric out comes, spearman rank correlation analysis was utilized.
DAT test
By use of CAT method DAT test was performed. The main objective of the experiment was to detect the anti-D that was bound with red cells. The DAT test was performed with every experiment.
IAT test
The IAT TEST was performed. It was aiming at discovering the presence of anti-D, as well as anti-C presence. The presence of either anti-D or anti-C, and this was used to determine the use of either of R2R2 in place of R1R1. The test was applied with serum samples 3, 4,5 and 6 by means of cells 6, as well as 7 that were obtained through the CSL panel sheet. In the AIT test, there were various test reagents that were used and these included; LISS, AHG reagent and also AHG control cell.
Titration
The four different models were acquired with explicit titration. On the other hand, the titration was used with two of the serum samples.
Standard curve
To 50 ul of 1% red cell suspension, 50 ul of the ten diverse dilutions of anti-D that is from 0.125 to 10ul/ml in the LISS with 5% BSA were added (12). The mixture was then incubated for 30 minutes at 37 degrees where it was shacked every five minutes. The mixture was washed in saline three times. 50 ul of Fab fragment antibodies was added to the mixture after which the mixture was incubated for thirty minutes at 37 degrees. Alternatively, to 50 ul of AHG FITC a different blend was added and incubated for thirty minutes at 25 degrees. The aim of this was to check for agglutination. Both the mixtures after incubation were washed three times in the saline. The mixture of Fab fragment antibodies was resuspended in 1ml of LISS/5% BSA, while the mixture of AHG FITC was resuspended in PBS/2% BSA. Subsequent to the resuspension, the mixtures were transferred to fluorescence tubes. The suspensions were later run by flow Cytometry where the target was to tally 50,000 cells for every sample that was examined.
Measurement of MFI
To the washed red cells, various dilutions of anti-D in PBS with 2% HSA were added after which they were incubated for 30 minutes at 37 degrees. The red cells were then washed three times in PBS after which FITC-labeled anti-IgG that had been diluted 1/20 in PBS with 2%HSA were added and the mixture and the mixture was nurtured for 15 minutes at 20 degrees. The cells were afterward scrutinized microscopically. On washing the red cells in PBS for three times, they were suspended in 2 ml PBS with 2% HSA. Part of the mixture was transferred to a glass tube and PBS with 2 % HSA added. The resultant dilution was found to be most favorable for the flow cytometry process giving a count rate of about 1,000 cells per sample. A sample of this suspension was examined in a flow cytometer to a point where 50,000 cells were calculated (13). The MFI was then measured in each one of the samples.
Results
Standardization
Different phenotypes were employed in the process where three standard curves were attained. The phenotypes used included: – OR1R1, OR1r and OR1R. To perceive the lowest level of the curve, a sample was run devoid of adding the CSL anti-D for each of the experiments.
In the first experiment, to establish a standard curve various dilutions of CSL anti-D were used, as well as OR1R1 cells. This experiment was run using both AHG IgG-FITC antibodies in stipulations of secondary antibodies. Count cell for AHG IgG-FITC was found out to be 19,000 and 36,000cell, while that of Fab fragments was found to be 5000 cells. The climax shape of Fab fragment and AHG IgG-FITC was smooth and disperse correspondingly. On the other hand, the negative model reading in MFI was 654, while the values with Fab fragment reagent were between 1200 and 15000. All of the obtained values in this test were located on a line except for one value which was unacceptable and therefore it was done away with (14). The outcomes of the experiment are shown in the graph below:
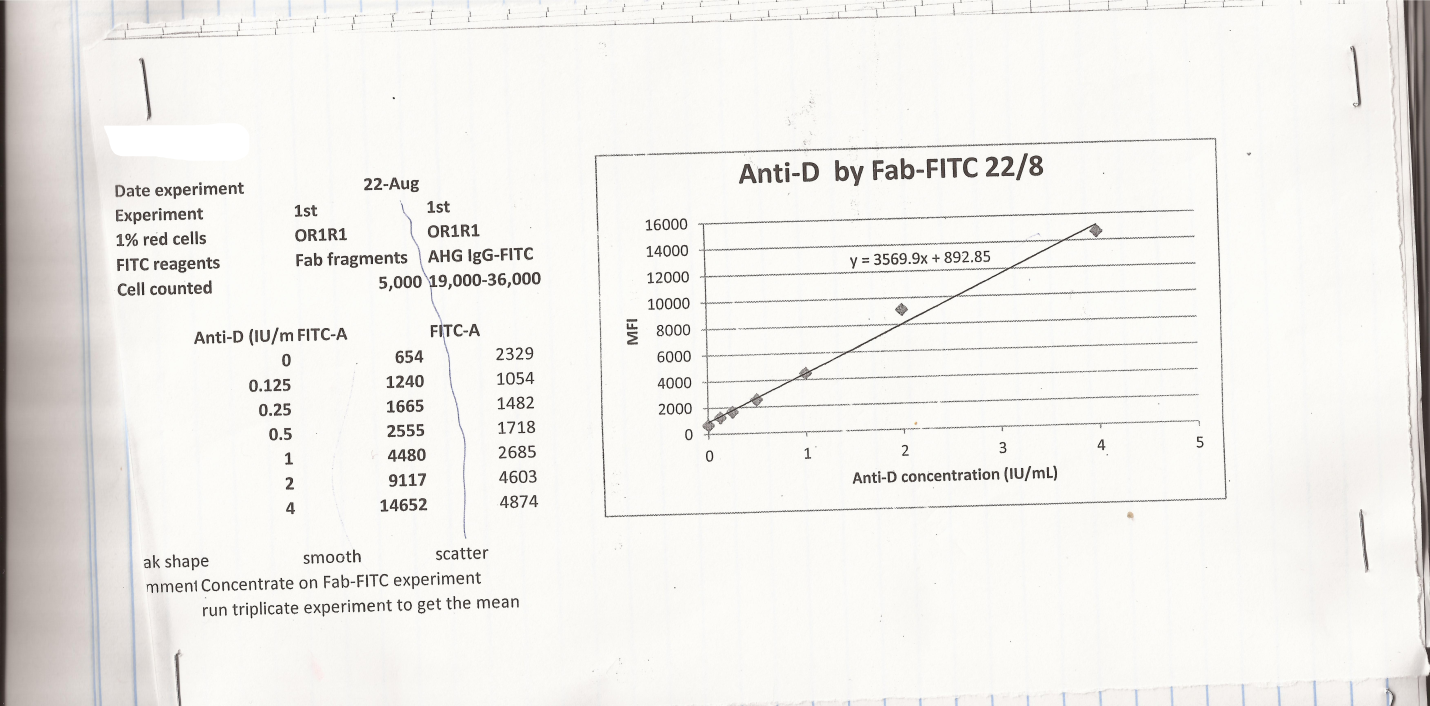
In the second experiment, different dilutions of CSL anti-D were used. 1% OR1r cells, as well as Fab fragment antibody, were employed in this test. The experiment was performed in triplicates in a flow cytometry where 20,000 cells were counted. The average value for MFI value was found out to be 600 to 1400. The value for the uppermost concentration was disqualified for the reason that it was not on the line.
The third and the last curve was found to be the most suitable. The dilutions that were used in this experiment included Fab fragment, 1%OR1R1, CSL, as well as anti-D. In order to provide precise results, the experiment was done in triplicates, and the average of the results obtained calculated. The MFI values of the various dilutions were found out to be between 900 and1400 and were all located on the line. In addition, to this negative control was also carried out in this test.
In the experiment, the age of red blood cells was also considered as expired red cells, and they were found to give haemolysed solution (15). Fab fragment duration before it was used, was also considered that the Fab reagent could not be used one week after being prepared, as it was found to be affected by light and, in response to this, freshly prepared Fab reagent was employed in the experiment. On preparation of new, as well as fresh reagents were prepared an ideal curve was attained.
Titration was done on serum sample 1 and the dilutions that were analyzed that is 1:16 to 1:128. The results obtained were applied to the third standard curve. Since the end results were not with the dilution factors, the samples also had to be done in duplicate using R2R2 cells in order, 1:16, 1:4, as well as 1:8 ratios. The outcomes were more persuasive where they were 13.22, 12.45, 13.45 and also 18.87 lu per ml for dilution 1:16, 1:32, 1:64 and 1:128 correspondingly. This was due to the fact that on titration the dilution factors were not essential and more so they interfere with the process. On running sample 2, a very low reading were obtained when they wee run by both R2 R2 and also by R1 R1. The outcomes obtained at this experiment were below detectable levels (16). For samples, three to six the dilutions were 1:16, 1:16, 1:32,768, as well as 1:64 correspondingly as they were attained. Since the concentration of anti-D was very high, thus more dilutions had to be done to sample 3. Negative results for IAT test for samples 3, 4 and 5 came as a result of the samples lacking anti-C, as well as anti-E, and containing anti-D. Sample 6 was found to produce a positive outcome an indication that it contained either anti-E or anti-C therefore; sample six was run in flow cytometer with R2 R2 cell, while the others that are sample 3, 4, and 5, were run with R1R1cells. Anti-D concentration in sample 3 was too small or unreadable. The sample 4 results were alternate and more than the efficient intensity. Sample 5 indicated a high concentration level of anti-D for instance 769.07lU per ml with a dilution ratio of 1:500. Sample 6 produced reasonable, as well as readable results.
The table above shows the AIT test results of sample 3, 4, 5 and 6. The samples that indicated negative result had been run with R1R1 cell while the sample with positive result had been treated with run with R2R2 cells.
Discussion
Considering the results presented in the experiments presented above, we can conclude that the above obtained results; the initial practice with Fab fragment antibodies to set up the standard curve was acceptable with only 5000 cells counting. On the other hand, for more consistence out comes the test had to be done in triplicates thus to increase the precision. Due to the fact that the cell count was not steady and at the same time the values were not in order, the standard curve could not be set up form the trial of AHG-FITC. In response to this for precise results in the test, Fab fragments antibodies were used in place of both. In the second trail a more precise data was obtained hence making it easier to come up with a standard curve. This was obtainable since all the values were situated on the line except for one value. The reason for this is that the reagents used had been prepared on the same day indicating that they must have been fresh and therefore lead to production of better results hence, the values of AHG IgG-FITC were not up to the standard for the required curve.
In the second test dissimilar dilutions were used to run the experiment that is CSL anti-D (0.25-6lU/ml). Fab fragment antibodies as well as 1%OR1r cells were employed in the experiment. For better and precise result the test was done in triplicates in flow cytometry and a count of 20,000 cells were counted (17). The mean value for MFI values was found to be 600-1400 due to the reason that the uppermost concentration value was not in line it was disqualified. After it was barred it was possible to come up with the intended standard curve. Flow cytometry is an undemanding method to carry out and thereby has the potential to put back the autoanalyzer. Differences in the two techniques that is autoanalyzer and flow cytometry point out that additional validation of the flow cytometric process is necessary prior to routine application. The flow cytometry process for quantification of anti-D employs the principle of light excitation, light scattering as well as flourochrome molecules for data production from cells and particles.
On the third trail 1%OR1R1, CLS anti-D as well as Fab fragment were utilized leading to production of a standard curve that turned out to be satisfactory. The test was done in triplicates for better and precise result and their means were calculated. In this experiment there were no values that needed to be disqualified since all the MFI values of the various dilutions fall on the line. This experiment was concluded to be the most suitable of all the three ones.
On reacting R1R1 cells with anti-D concentrations of the different dilutions of sample 1 were not parallel with the dilution factors of the standard curve. The reason for not being parallel could be combination of anti-D with either anti-E or even anti-C. This made the R1R1 to be substituted with R2R2 for the anti-C or anti-E to be kept out with an aim of coming up with good quality results that is to get outcomes that were below the noticeable levels. The cause of this was due to the fact that anti-D being low as well as due to an unintended fault in the grounding. There were also inexplicable outcomes with sample 4 since the appraisal of flow cytometry for all dilutions were beyond the precise concentration.
The relative study of the various flow cytometric techniques for assessing anti-D concentrations, verified disparity in the results attained for some certain serum samples. The grounds for the differences can be accredited to either the characteristics of the serum antibodies or to dissimilarities in the methods which are examined. The interassay of below 10% for the standard curve indicated that each of the techniques had high-quality reproducibility with a distinct consistency of antibody preparation. On the other hand, a closer analysis of the there standard curves acquired in the trials places interests on some of the dissimilarities in the techniques used. Antibody incubation that is shortened can account fro the shift to the right of the standard curves. The move can also be accredited to either:
- The use of PBS as the diluents
- Lesser fluorescein:protein ratio of the FITC-labeled anti-human IgG reagent
- High cell concentrations
The considerable disparity between the first curve of method one and curve three was due to a small antibody concentration range as well as the high cell number employed in the first method of the standard curve. A setback linked with a standard curve over a small concentration range is the intricacy in deciding the correct sample dilutions to allow interruption. In this study serum samples were examined before use in order to find out the most favorable dilutions for the interpolation alongside the standard curve. The various anti-D concentrations resulting from the flow cytometric techniques for every serum sample showed the intricacy of the relation of the target cell and the serum samples.
The divergences between the autoanalyzer derived values and those of flow cytometer cannot be ascribed to various antibody specificities of the reagent cell type or the serum model. The presence of either anti-E or anti-C component generated high values that made them be substituted with R2R2 so as to keep them out for a quality standard curve (18). With a number of serum samples the prevalence of diluent for cells and the serum influences the value of anti-D acquired. The disparities in antibody composition as well as the heterogeneous assortment of affinities viewed in serum samples may be reflected by the fact that the selection of diluents influences the value acquired. The utilization of low ionic salt solution all through the flow cytometric test notably boosts the observed concentration of anti-D, although the resulting value can not be compared to that of the autoanalyzer. When low ionic salt solution is employed as a diluent instead of PBS the uptake of anti-D is superior and at the same time this disparity can be further improved with BAS/ low ionic salt solution As a result the antibodies that are normally uncreative at low concentration are detectable with low ionic salt solution. Serum-to-packed-cell ratio has an effect on the quantity of antibody bound per cell. The final quantity of antibody bound per cell is as well affected by the temperature of serum samples with antibodies with assorted equilibrium contrasts
In the comparison of the flow cytometric derived anti-D concentration with that obtained autoanalyzer is considered to be inappropriate. An additional way to prevent agglutination is to dilute the anti-D antibody to concentrations where the amount of antibodies for each red cell required for agglutination is not surpassed.
In the autoanalyzer technique, the measurement of antibody bound cell done through enhancement of cell lysis in concert with agglutination as well as the hemoglobin absorbance, while in the flow cytometric process the measurement is done directly. It is for that reason likely that the divergence in anti-D values for a few sera simply reflects the disparities in the behavior of antibodies in the two techniques. The differences in serum anti-D concentrations resolute by an enzyme-linked immunosorbent assay as well as by the autoanalyzer have also been described. The flow cytometry process perceives the only the IgG isotype of anti-D antibodies, it also measures the sensitization of the red cells and this involves the binding of the antibodies to the consequent antigen. On the contrary, the autoanalyzer hemagglutination process engrosses the risk for this purpose high values via including the IgM capacity.
Conclusion
In conclusion, the flow cytometry was found suitable to measure the quantity of anti-D antibodies in sera of immunized women. Flow cytometry is an appropriate method and as well a highly reproducible technique for the quantification of anti-D. Autoanalyzer is suggested to be an alternative to flow cytometry although the autoanalyzer technique is an extremely responsive for variations in the ionic strength in the already utilized solutions which can be substantial. In the flow cytometry this kind of practice is discrepancy is in attendance. The main difference between the two techniques is the kind of secondary antibody, in flow cytometry agglutination of sensitized red cells is a likely predicament as it gets in the way with the measurement on single cells.
For both practical as well as economical advantages, flourochrome-conjugated IgG molecules are used in the secondary step, in place of Fab fragments. However, this would lead to some drawbacks such as the Fab fragments which are usually conjugated with a small number of FITC molecules hence resulting in a reduced fluorescent signal. As a result, intensification of the system is therefore essential, together with the use of low ionic salt solution (LISS), low concentration of the red cells and prolonged incubation of the Fab fragments. The utilization of Fab anti-IgG does not in any way keep out the likelihood of agglutination caused by IgM which can be there even if perceived. Furthermore, the cost of conventional whole IgG molecules is much less as compared to the Fab fragments. An additional way to prevent agglutination is to dilute the anti-D antibody to concentrations where the amount of antibodies for each red cell required for agglutination is not surpassed.
Quantification of anti-D by flow cytometry is greatly influenced by the serum antibody characteristic and also by the technique used. It would be right to conclude that flow cytometry is an unchallenging method to carry out and therefore it has the potential to put back the other related method that is the autoanalyzer technique. A flow cytometer for the quotation of anti-D usually has an exceptional feature in that it measures fluorescence per cell or particle. The anti-D quantification by flow cytometry is normally prejudiced by the serum antibody distinctiveness as well as by the process utilized. There are three available flow cytometric techniques that are aimed at anti-D quantification. Each and every one of these methods uses diverse cell sensitization as well as different antibody recognition circumstances (19). The variation in antibody detection and also in cell sensitization of the various techniques may lead to a wide range of outcomes which will in its turn call for a timely direct contrast of the above techniques. The major differences between the flow cytometry and the autoanalyzer methods point out that more justification of the flow cytometry is needed prior to the usual exploitation.
The quantification of Rh antibodies is clinically significant in foreseeing the jeopardy of the hemolytic disease of the infant and therefore it is important that the various techniques towards the quantification of anti-D are thoroughly and carefully looked into so as to increase the detection of such diseases as well as reduce death in the new born. This would therefore call for introducing the techniques to the scholars so as to ensure that the needed information is nurtured. With the development of such techniques in the field of medicine, a lot of advantage will come along with it as the flow cytometry has a vast number of applications that are essential in the field as well as vital for a human life. Some of the applications of the flow cytometry in the laboratory engross the diagnosis monitoring as well as prognosis of diseases. Other medical applications of the flow cytometer include; – investigation of lymphoma and leukemia detection of negligible residual disease that is a disease that is past the extent of morphological recognition by the use of conventional microscopy, autoantibody detection, in HIV infection, in T cell cross-match, in foeto-maternal hemorrhage, in reticulocyte scrutinization, in platelet function and counting, in immunodeficiency illness as well as in stem cell inventory (20). The flow cytometry technique for the quantification of anti-D is therefore of great value not only to the antibodies but also to other areas in the medical field. It helps in the detection of diseases as well as in the prediction of an infection.
Acknowledgement
This study was is held up by a funding (72/112) from the County Council United kingdoms
References
Ardnt P.A. Applications of flow cytofluorometry to red blood cell immunology.1999
Bell A, Shenton B, Garner G. The flow cytometric crossmatch in solid organ transplantation.1998.
Mandy F, Janossy G. T-cell subset counting and the fight against AIDS.2002.
Bergeron m. Affordable CD4 T-cell enumeration for resource-limited regions.2008.
Campana D, Coustan-Smith G. Minimal residual disease studies by flow cytometry in acute leukemia.2004.
Davis B.H, Bigelow N.C. Reticulocyte analysis and reticulocyte maturity index. In: Flow Cytometry.1997.
Freeman J. Applications of flow cytometry in transfusion medicine.1995.
Foon K.A, Craig F.E. Flow cytometric immunophenotyping for hematologic neoplasms. 2008.
Garratty G. Applications of flow cytofluorometry.1995.
Gratama J.w. Flow cytometric enumeration of CD34+ hematopoietic stem and progenitor cells.1998.
Harferlach T, Kern W. Monitoring of minimal residual disease in acute myeloid leukemia.2008.
Harrison p, Charlie L. Platelet counting by the RBC/platelet ratio method.2001.
Michelson, A.D. Evaluation of platelet function by flow cytometry. 2006.
Owens M.A. Validation and quality control of immunophenotyping in clinical flow cytometry.2000.
Shanahan T. Application of flow cytometry in transplantation medicine.1997.
Segal H, Briggs C. ) Impact of immunological platelet counting (by the platelet/RBC ratio) on hematological practice. 2005.
Weir K, keeney M. Single platform flow cytometric absolute CD34+ cell counts based on the ISHAGE guidelines. 1998.