Introduction
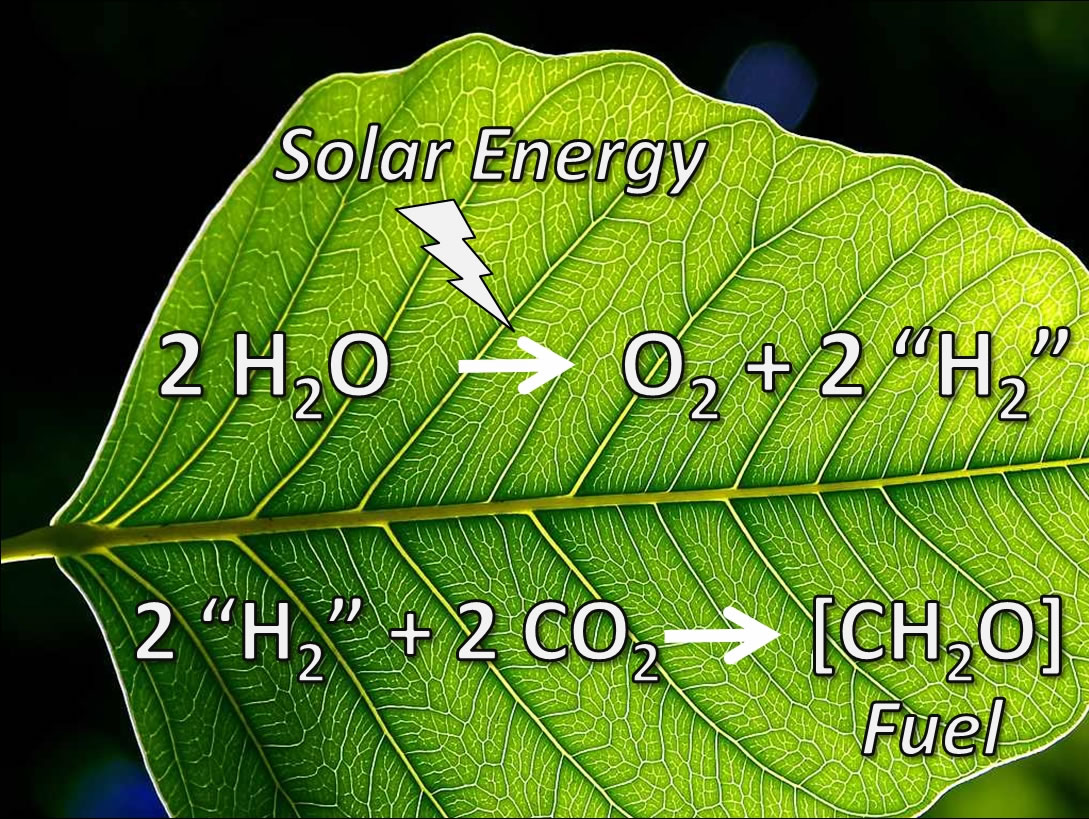
The natural photosynthesis process changes light energy into chemical energy. However, in recent times, there are ongoing active studies in the field of artificial photosynthesis. Chemical energy originates from the breakdown of CO2 and H20 under light energy. The process results in the production of carbohydrates for plants alongside oxygen that is aerobic in living organisms. However, the minor details of the energy-transformation processes at the molecular level remain unclear.
Recent Advances
Latest advances in specific areas of spectroscopy, crystallography, and molecular genetics have provided new opportunities for scientists to explore artificial photosynthesis. Specifically, scientists strive to use the known and change them into “functional, efficient, synthetic systems that will tap the endless supply of energy coming from the sun”. Researchers believe that artificial photosynthesis can work on a large scale and generate energy to serve the needs of human. An example of the possible exploitation of artificial photosynthesis to generate energy is the use of photovoltaic (PV) cells. However, researchers have cited high costs and the occasional absence of the sun as limiting factors to harvesting solar energy.
The demand for clean energy has pushed many researchers to focus on artificial photosynthesis as a possible source of clean energy that can replace fossil fuels. Thus, artificial photosynthesis could be the solution to the world’s energy challenges.
Michael Berger observes that recent approaches focus on titanium dioxide as a possible photocatalytic material due to its relatively “low cost, chemical stability, and photostability”. However, the availability of the ultraviolet (UV) limits the application of this technology on a large-scale (about four percent of the world’s region has solar UV). This makes the technology impractical on a large scale.
Other researchers have focused on the exploitation of tungsten trioxide as new photoanode material. Still, others have considered tungsten trioxide as “a mixture material with titanium dioxide for splitting water because it can offer relatively small bandgap (∼2.5 eV) and corrosion stability in the aqueous solution”. While tungsten trioxide has exhibited a major potential in artificial photosynthesis, its quantum remains low. A new study with titanium oxide nanotubes with tungsten oxide has shown a significant improvement in artificial photosynthesis.
Meanwhile, there are ongoing efforts to use varied tungsten trioxide or titanium dioxide to boost the effectiveness of electrochromic impacts and photocurrent in water solutions. Some scientists at the University of Texas have shown that carbon with elements of titanium dioxide (TiO2) nanotubes have abilities to enhance photocurrent densities. Still, these researchers also used “titanium oxide nanotubes with tungsten oxide (WO3) as photoanode” to demonstrate solar harvesting techniques. The result indicated that such nanocomposite materials were effective and stable methods of trapping solar energy.
Challenges
Researchers have faced many challenges despite their numerous contributions to artificial photosynthesis. These challenges are mainly critical science and financial issues. However, this is an emerging technology. Therefore, artificial photosynthesis still has many challenges to conquer in the future before it can be commercially viable.
In the US, in 2011, President Obama designated funds for the Joint Center for Artificial Photosynthesis (JCAP), which consists of Berkeley Lab and Cal Tech. Researchers have concentrated on imitating chloroplast and enhancing its operations. Chlorophylls can retain sunlight and facilitate water breakdown processes efficiently. Several catalysts facilitate the photolysis process. In addition, there is a chloroplast that has elements, which can transfer electrons, detach hydrogen ions for the adenosine triphosphate process, and maintain the arrangement of the cells. This is what many researchers aspire to imitate.
For instance, JCAP scientists want to develop various parts and join them to form a prototype for “scalable and cost‐effective solar fuel generators without the use of rare materials or wires and robustly produces fuel from the sun that is ten times more efficient than typical current crops”.
The major issue for cells that absorb light is the caustic nature of aqueous elements when reactions take place. In addition, nanophotovoltaic (nanoPV) cells cannot stick to catalytic surfaces properly. Researchers at the Berkeley Lab have developed both organic and inorganic PV elements in order to enhance charge transfer, durability, and efficiency.
In addition to existing challenges, another major obstacle is the development of an effective catalyst for artificial photosynthesis. The catalyst should enhance reaction, electron portability, and should not be from rare and expensive metal like platinum. Moreover, such catalyst should be long lasting and able to withstand rapid reaction that involves photooxidation. Still, such a catalyst should have the ability to yield the maximum chemical energy from low activities. Such challenges have pushed scientists to develop new approaches, which can efficiently control the catalyst and account for the entire chemical reaction that occurs.
The future research should focus on developing advanced methods of enhancing nano structures and various coating elements, which have low band gap and are stable during reaction and in aqueous medium.
Artificial photosynthesis now works
Most scientists have dedicated their resources to enhance the effectiveness of the photosynthesis process through artificial photosynthesis. Artificial photosynthesis has attracted the attention of all researchers across developed nations. In the past, these researchers have achieved mixed results. The challenge has been developing an effective catalyzer for facilitating the process of artificial photosynthesis. Such a catalyzer can allow scientists to overcome challenges in achieving functional artificial photosynthesis.
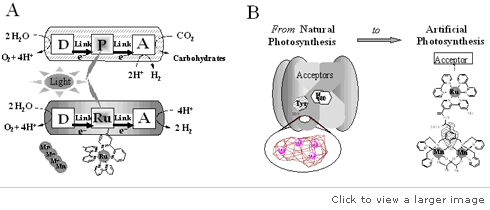
In Sweden, a group of researchers from the Royal Institute of Technology in Stockholm developed “a molecular catalyzer that could oxidize water quickly”. These researchers reported that their advances had achieved “over 300 turnovers per second with the newly equipped artificial photosynthesis”. They claimed that the natural photosynthesis could achieve between 100 and 400 turnovers per second. Therefore, their molecular catalyzer remained exceptional.
These scientists believe that the outcome of their research has provided the groundwork for revolutionary development in artificial photosynthesis. The development of a catalyzer can transform many aspects of energy production. For instance, the catalyzer can produce hydrogen in mass, allow express changes of solar energy to hydrogen, or facilitate the generation of electric energy. The only challenge is how to develop a cost-effective catalyzer.
Scientists have also developed an artificial leaf during research in artificial photosynthesis. Artificial leaf has existed for a while. However, new studies have transformed materials and altered the process to develop economically viable projects.
The artificial leaf has “a nickel-molybdenum-zinc compound and cobalt film on different sides together with sunlight absorption materials” between the two compounds. The side with the “nickel-molybdenum-zinc releases hydrogen gas, whereas the side with the cobalt film releases oxygen gas during the photolysis”. The resultant gas generates the required electricity.
Daniel Nocera, a researcher at the Massachusetts Institute of Technology believes that the use of nickel-molybdenum-zinc element for generating hydrogen gas is an important development because it eliminates the need for a platinum catalyst, which is an expensive catalyst. Such materials are readily available and affordable. The new project also has longer and continuous operating hours of at least 45 hours than the previous version that could only go for barely a single day.
Researchers in artificial photosynthesis believe that artificial photosynthesis shall meet the energy requirements of the world in the future. The only challenge is how to make artificial photosynthesis cost-effective in the future.
Implications
Such advances in artificial photosynthesis have significant implications to researchers. For instance, the ability to generate hydrogen gas from inexpensive shall allow for mass production of the gas for generating electricity. As new ideas emerge, artificial photosynthesis would provide alternative energy or replace fossil fuels. Therefore, funding research in artificial photosynthesis is necessary for solving global energy needs.
Conclusion
The fundamental research has provided technologies that allow us to comprehend the intricate photochemistry that involves the normal photosynthesis. Still, recent advances in artificial photosynthesis show that researchers can develop methods of generating energy from photovoltaic solar cells.
Such advances have shown steady progress as researchers seek available and affordable catalysts for generating hydrogen. Thus, the major challenges to artificial photosynthesis and its implications are funds and research development. Therefore, scientists must make gains in the laboratory real by designing “solar fuel generation systems with the required efficiency, scalability, and sustainability to be economically viable in order to create a breakthrough that could have a revolutionary impact on humanity’s energy systems”.
Reference List
Addison, G, FB Marcia, AB Barbara, and E Edward, Science at the frontier, National Academy Press, Washington, D.C.,1992.
American Chemical Society, Secrets of the first practical artificial leaf, Science Daily, 2012. Web.
Bapna, M, A Breakthrough for Renewable Energy? Insight WRI, 2012. Web.
Berger, M, Nanotechnology advances the efforts to achieve artificial photosynthesis, Nano Werk, 2006. Web.
Charlie C, C Jung and S Dong, Clean Energy from Simulating the Leaf: Artificial Photosynthesis, Berkeley, 2012. Web.
Matthew, TM, Y Lin, G Yuan and D Wang, ‘Forming Heterojunctions at the Nanoscale for Improved Photoelectrochemical Water Splitting by Semiconductor Materials: Case Studies on Hematite’, Accounts of Chemical Research, 2013.
Nida, R, and I Lee, Artificial Photosynthesis Now a Reality, Berkeley, 2012. Web.
Nocera, DG, ‘The Artificial Leaf’, Accounts of Chemical Research, vol. 45, no. 5, 2012, pp. 767–776.
Osterloh, FE, ‘Inorganic nanostructures for photoelectrochemical and photocatalytic water splitting’, Chemical Society Reviews, vol. 6, no. 42, 2013, pp. 2294-2320.