Abstract
Retinol is one of the vitamins that the body requires for normal growth, development, and functioning. Chemically, retinol has a molecular formula of C20H30O, which forms a beta-ionone ring and isoprenoid chain with a functional alcohol group (-OH) at the terminal. The physical properties of retinol are that it is a crystalline yellow-orange solid at room temperature with melting and boiling points of 62-64oC and 137-138oC respectively. As a chemical property, retinol undergoes oxidation to form retinal and retinoic acid. Both plants and animals are sources of retinol as they produce carotenoids and preformed retinol accordingly. Although retinol is important in vision, regulation of translation, and dermatological use, excessive intake of retinol predisposes one to osteoporosis, cancer, congenital effects, and hormonal imbalance.
Introduction
Retinol is an organic molecule that plays a central role in regulating physiological activities in the body. The main function of retinol is the production of visual pigments such as rhodopsin that enhances the visual capacity of an individual in darkness (Zhong, Kawaguchi, Kassai, & Sun, 2012). Given that retinol is a form of vitamin, it mediates metabolic processes that are essential in the growth and differentiation of cells, embryonic development, hematopoiesis, growth of epithelial cells, and the functioning of the immune system. Essentially, retinol is an alcohol derivative of vitamin A, which is an active molecule. Thus, this essay examines the chemical composition and structure of retinol, its physical and chemical properties, sources, benefits, and adverse effects on the human body.
Chemical Composition and Structure
Retinol is an organic compound that is compost of carbon, hydrogen, and oxygen. Essentially, retinol comprises of 20-carbon atoms, 30-hydrogen atoms, and an atom of oxygen. The molecular formula of retinol is C20H30O. The elements that are present in retinol combine to form a beta-ionone ring and isoprenoid chain, which are main the main components of retinol. The functional group of retinol is an alcohol group (-OH), which is attached at the terminal end of the isoprenoid. Moreover, retinol exists in all-trans form as shown below.
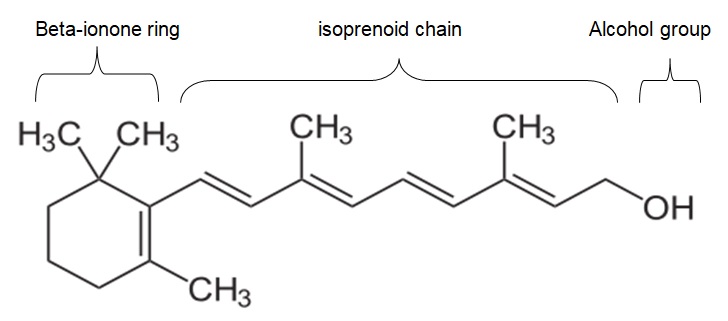
Physical and Chemical Characteristics
Retinol is a light molecule that has a molecular weight of 286.45g. Its melting point is between 62-64oC, while the boiling temperature is between 137-138oC. This means that at room temperature and pressure, retinol exists as solid. Retinol is a crystalline solid that appear yellow or orange in color. The density of retinol is 0.954g/cm3, which means that it is lighter than water. Since retinol is an organic molecule, it is insoluble in polar substances such as water and glycerol, but they are soluble in non-polar substances such as alcohol, chloroform, ether, and oils.
As chemical properties, retinol can undergo oxidation into retinal and retinoic acids. In this view, retinol is unstable at room temperature and in the presence of air. Therefore, it is extracted and stored under the presence of protective anti-oxidant. Moreover, retinol can react with tricholoacetic acids, antimony trichloride, and trifluoroacetic acids to give colored substances.
Material Presence
Retinol is present in both plant and animal foodstuffs. Among plants, retinol is present in carrots, kales, spinach, tomatoes, pumpkin, sweet potato, broccoli, and mango amongst others. In animals, retinol is present in liver, eggs, whole milk, and fish. Animals provide preformed retinol while plants provide provitamin A. Preformed retinol is available as retinyl esters as it is complexed with esters, whereas provitamin A is present as carotenoids. Metabolism of retinyl esters and carotenoids produces retinol, which is an active molecule in the body.
Uses
Given that retinol is a vitamin, it mediates many functions in the human body. One of the important functions of retinol is to enhance photoreception. Retinol usually binds to proteins called opsin in the eyes to form rhodopsin. The periodic isomerization of cis-retinol into trans-retinol induces optical signal, and thus enhances vision during dark. Thus, people who experience difficulties in their vision take retinol supplements to improve their vision ability. Retinol is also important in gene regulation because cells take up retinol and oxidize it using retinol dehydrogenases into retinoic acid and retinal. According to Zhong, Kawaguchi, Kassai, and Sun (2012), retinoic acid regulates translation of proteins while retinal inhibits adipogenesis. Another important use of retinol is in the formation of body creams, which are applicable in softening the skin. Fundamentally, retinol enhances the differentiation of keratinocytes in the epidermal layer, and thus aids in the removal of wrinkles and acne.
Adverse Effects
Although retinol is beneficial to humans, excessive intake causes toxicity in the body. Zhong, Kawaguchi, Kassai, and Sun (2012) state that excessive intake of retinol overwhelms and bypasses metabolic pathways, and thus causes toxicity. The excess retinol in the body accumulates and causes adverse reactions, which affects physiological and metabolic functions of the body. In the body, retinol dehydrogenase oxidizes retinol into retinal and retinoic acid, which have a detrimental effect on metabolic processes and physiological mechanisms. Common symptoms associated with retinol toxicity are irritability, anorexia, hair loss, headaches, blurry vision, altered mental status, and abdominal pain amongst others. In essence, retinol toxicity causes osteoporosis, congenital defects, cancer, hepatic injury, and hormonal imbalance.
Conclusion
Retinol is an important vitamin because it mediates physiological and metabolic mechanisms in the body. It is a crystalline yellow-orange solid that is present in plants and animals as carotenoids and preformed retinol respectively. Retinol is composed of the beta-ionone ring and isoprenoid chain with a functional alcohol group (-OH) at the terminal. Retinol is important in vision, regulation of translation, and treatment of skin. However, excessive intake predisposes one to osteoporosis, cancer, congenital defects, hormonal imbalance, and hepatic injury.
Reference
Zhong, M., Kawaguchi, R., Kassai, M., & Sun, H. (2012). Retina, Retinol, Retinal, and the Natural History of Vitamin A as a Light Sensor. Nutrients, 4(12), 2069-2096.