Introduction
The chemical formula for acetone is (CH3)2CO. It is an organic compound, which is a flammable, mobile, and colorless liquid. Acetone is the simplest form of the ketones. It is miscible with water and it acts as one of the best solvents. Generally, acetone is used as solvent for cleaning purposes in most laboratories (Young 2001). In 2010, over 6.7 million tonnage of acetone was produced across the globe. Most of the acetone was used to make bisphenol A and methyl methacrylate. Besides, some acetone was used as a solvent. Other domestic uses of acetone include removing nail polish. Nail polish remover contains “acetone as one of the active ingredients and it also acts as paint thinner” (Young 2001, p.1156). Acetone is very crucial in the field of organic chemistry.
Conventionally, acetone occurs naturally during metabolism in human beings. The compound is normally prevalent in urine as well as in blood. Individuals suffering from diabetes produce acetone in large amounts. “Reproductive toxicity tests prove that acetone has low chances of causing any reproductive complications” (Young 2001, p.1170). Indeed, nursing mothers, pregnant women, and children produce a lot of acetone. Their bodies require a lot of energy, thus leading to high levels of acetone production. Bahl, Andersch, and Gottschalk (2000) posit, “Ketogenic diets that increase acetone in the body are used to reduce epileptic attacks in infants and children who suffer from recalcitrant refractory epilepsy” (p. 202).
Acetone (dimethyl ketone, 2-propane, CH3COCH3) has a chemical formula mass of 58.079 and it is the most crucial and the simplest of all the ketones. Moreover, it is a combustible, colorless, and mobile liquid with a slightly sharp and scented smell. In all proportions, “acetone is miscible with water and organic solvents like methanol, ether, esters, and ethyl alcohol…Acetone works as a solvent for nitrocellulose and cellulose acetate” Young 2001, 1163). Additionally, it is used to carry acetylene. As a raw material, acetone helps to manufacture a number of products like Methyl methacrylate, ketene, isophorone, diacetone alcohol mesityl oxide, bisphenol A, methyl isobutyl ketone, and many others.
Manufacture of acetone
Acetone is manufactured in different ways. Among the ways, include:
- Dehydrogenation of isopropyl alcohol.
- The Cumene hydro peroxide process for phenol and acetone.
- Direct oxidation of hydrocarbons.
- Catalytic oxidation of isopropyl alcohol.
- Acetone is also obtained as a by-product during the propylene oxide process employed by oxirane.
- The p-Cymene hydro peroxide process for p cresol and acetone.
- The Diisopropylbenzene process for hydroquinone or resorcinol and acetone.
Physical and chemical properties of acetone
- Appearance: Acetone is a colorless liquid.
- Molecular weight: the compound has a molecular weight of 58.079
- Color: acetone is colorless.
- Acetone has a specific gravity (g/ml):- 0.79 at a 20° C
- The melting point of acetone is -94.6° C
- It has a boiling point of 56.13° C (at 760 mm Hg)
- At 20°C, acetone has a vapor pressure of – 24.7 KP
- Volatile by vol. (%):- 10
- Solubility description: acetone is miscible with water and other solvent s with a solubility value of (g/100g H 2O20°C ):- 100
- Flammability limit (lower) (%):- 2.1
- Auto Ignition Temp. (°C):- 540
- Flammability limit (upper) (%):- 13.0
Reactivity and stability
Acetone is stable under the ordinary conditions of use. Nevertheless, one needs to handle it with care and to avoid contact with acids and strong oxidizing agents. Besides, acetone is highly combustible and it should not be kept near flame, heat and other sources of detonation. In addition, one ought to ensure that acetone does not come into direct contact with materials like sodium hydroxide, potassium sulphate and acids like nitric acid and sulphuric acid. Thermal breakdown of acetone may lead to the release of perilous gases such as oxides of carbon (Young 2001).
Uses
According to Lozano, Yip, and Hanson (1992), “a third of the world’s acetone is used as solvent, and quarter is consumed as a precursor to methyl methacrylate” (p. 369).
Solvent use
Acetone acts as the “best solvent for most synthetic fibers and plastics used in making laboratory bottles such as polycarbonate, polystyrene, and other types of polypropylene…is also suitable for thinning fiberglass resins, dissolving superglue and two-part epoxies before hardening” (Behrend et al. 2008, p.75). Besides, it is suitable for cleaning tools made of fiberglass. Moreover, acetone is used as a “volatile constituent of various varnishes and paints; in addition it is used in preparing metals before painting them since it is a heavy-duty degreaser…Additionally, it facilitates to thin adhesives, vinyl, and polyester resins” (Behrend et al. 2008, p.75). Acetone is used in soldering applications. It facilitates to remove solder rosin after completing the soldering process, which aids in avoiding chances of rust corroding the soldered materials.
Storage of acetylene
Acetone is highly flammable. Nevertheless, it acts as a solvent to facilitate in “the storage and transportation of acetylene, which is hard to pressurize safely as a pure compound…to transport acetylene, porous materials are put into a container and the container filled with acetone” (Behrend et al. 2008, p.84). Acetylene is then introduced into the container, which dissolves in acetone thus facilitating in its storage and transportation.
Medical and cosmetic uses
Acetone is used in a number of cosmetic and general medical applications. Besides, it plays a significant role as a food additive and in food packaging. Acetone plays a significant role in chemical peeling. “Salicylic acid, trichloroacetic acid, 30 percent salicylic acid in ethanol, and glycolic acid are some of the common chemicals used in chemical peels today, while the skin is cleaned appropriately and surplus fat removed prior to chemexfoliation” (Wagner 1913, p. 28).
Laboratory uses
Darwent et al. (2000) claim, “In the laboratory, acetone is used as a polar aprotic solvent in a variety of organic reactions, such as SN2 reactions…Besides, as a solvent, acetone is crucial for the Jones oxidation… is also used to rinse laboratory glassware since it is cheap and highly volatile” (p. 1827). Nevertheless, when it reacts with water, acetone does not form an azeotrope. It is possible to cool acetone to -78°C using dry ice. Acetone or dry ice baths help in reactions that require low temperatures. Under ultraviolet light, “acetone is fluorescent and its vapor is used as fluorescent tracer in fluid flow experiments” (Darwent et al. 2000, p. 1827).
Domestic and other uses
Acetone is in most cases one of the major constituents in cleaning materials like nail polish remover. Acetone is used as “superglue remover and it facilitates to remove residues from porcelain and glass” (Behrend 2008, p.54). According to Young (2001), “acetone serves as an artistic agent when rubbed on the back of a laser print or photocopy placed face-down on another surface and burnished firmly, the toner of the image transfers to the destination surface” (p.1154). One immerses the item in acetone and he or she uses stiff brush to remove the skin glue.
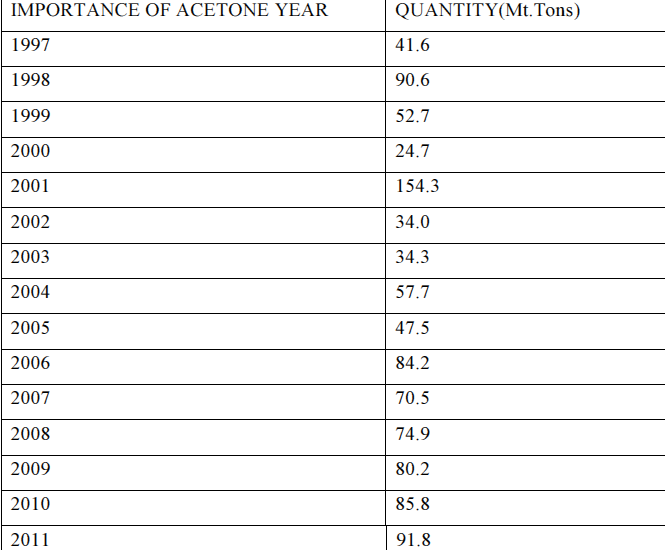
Previous supply and current demand
Most of the country’s acetone requirement is satisfied through importation. The table below was extracted from the Ethiopian Customs Authority. It reflects the importation of acetone between 1997 and 2011.
Methods of production
There are different methods of producing acetone. Among the methods include, catalytic dehydrogenation of isopropanol, oxidation of butanol, oxidation of isopropyl benzene, and oxidation of propylene among others.
Oxidation of propylene
Wagner (1913) notes, “Direct oxidation of propylene with air leads to the production of acetone” (p.36). During this process, a mixture of copper chloride and palladium chloride acts as the catalyst. The following represents the overall reaction
C3H6 + 1/2O2 → C H3COCH3
Oxidation of butanol
This process entails catalytic oxidation of n-butane using manganese acetate or cobalt to produce acetic acid. The process also leads to the production of other byproducts of commercial worth in patchy amounts. The oxidation reaction in the Celanese process takes place at a temperature that ranges from 150-225° C and an atmospheric pressure of approximately 505 atm. The following is the overall reaction.
CH3CH2CH2CH3 + O2 → CH3COOH + CH3COCH3
Co-product of Glycerine- H2O2 Process
When obtaining glycerine from propylene through acrolein, acetone comes out as a byproduct. The following is the overall reaction.
CH3CH═CH2 + H2O → CH3CHOHCH3 + O2 CH3COCH3 + H2O2
Oxidation of isopropyl benzene (Cumene)
The reaction between propylene and benzene produces Cumene. The subsequent oxidation leads to the formation of hydro peroxide. When split, hydro peroxide yields acetone and phenol and to obtain pure acetone and phenol the impure products are fractionated.
Dehydrogenation of isopropanol
Catalytic dehydrogenation of isopropanol yields acetone. ZNO acts as the catalyst in this process. In a bid to obtain pure products, one has to fractionate the crude products obtained from the reaction.
(CH3)2CHOH (CH3)3CO + H2
The acetone obtained in the reactor goes through the phase divider and next into a division system, which comprises single stripping and double distillation columns. A recycle stream channels a mixture of water and isopropyl alcohol (with traces of acetone) back into the mixer that supplies the reaction system. This process leads to the production of high-purity acetone and is in most cases applied in biomedical applications.
Reason for selecting the process
In many cases, people prefer to manufacture acetone from Cumene process to the dehydrogenation of isopropanol. Nevertheless, the catalytic dehydrogenation of isopropanol is preferred when there is a need of manufacturing high-purity acetone, particularly for use in the biomedical field. Through this process, about 88 percent of isopropanol is recycled, making the process the most cost effective. The process yields 99 percent pure acetone. During the process, isopropyl alcohol is introduced into the reactor, where it is heated and reacted over a catalyst at low atmospheric pressure. The following equations represent the reactions that take place in the reactor.
CH3-CHOH-CH3 → CH3-CO-CH3 + H2
Isopropyl alcohol (IP) Acetone (AC) Hydrogen (HY)
CH3-CHOH-CH3 + ½ O2 → CH3-CO-CH3 + H2O
Safety
Flammability
Acetone is highly flammable. At temperatures above its flash point (-20°C), acetone may explode or even trigger a flash fire. In vapor form, acetone might stream along the ground to remote ignition sources and flare back. Nevertheless, acetone requires high ignition energy, which prevents chances of accidental ignitions. Acetone has a high concentration of vapor. Besides, it has a high rate of evaporation (Young 2001). Hence, evaporation leads to a cooling effect around the liquid making it hard for acetone to ignite even if it is exposed to ignition sources. The liquid has to remain in contact with an ignition source for a long time. Acetone is “oxidized to produce acetone peroxide, a compound that is very unstable” (Behrend et al. 2008, p. 145). The oxidation may take place inadvertently like when solvents containing acetone mixes with hydrogen peroxide accidentally. The main reason why there is no extensive use of acetone peroxide is its instability.
Health information
After an extensive study, it was found that acetone does not have severe and lasting side effects if ingested or inhaled accidentally. However, if inhaled in large amounts, it might cause irritation of the throat or the eyes. Today, acetone is not considered as a mutagenic chemical or carcinogenic. Most consumer products contain acetone in small proportions. Indeed, acetone is classified among the generally recognized as safe (GRAS) substances when found in small proportions in baked food, beverages, preservatives, and deserts.
Toxicology
In normal use, acetone exhibits low level of toxicity, and there has never been any report of health effects where people observe the set precautions when using acetone. If inhaled in high amount, acetone might cause irritation and/or cause depression to the central nervous system. In addition, when exposed to the eyes, it causes irritation and it might pose risks to pulmonary aspiration. Acetone has anticonvulsant impacts in animal forms of epilepsy when consumed in small amounts.
Environmental effects
Acetone is readily available in both water and soil. It evaporates at a high rate and can last in the atmosphere for about 22 days. The ultraviolet light degrades it into ethane and methane through photolysis. Acetone dissolves slowly “in animals, soil, or in water bodies because at times, the microorganisms present in soil or water bodies consume it” (Darwent et al. 2000, p.1846). Nonetheless, acetone is one of the underground water contaminants since it is highly soluble in water. It poses a “high risk of oxygen diminution in aquatic life because of the microbial consumption” (Darwent et al. 2000, p.1846).
Conclusion
Acetone is one of the organic compounds that are highly flammable. It is a mobile and colorless liquid. It is miscible with water and acts as one of the best solvents. Generally, acetone is used as solvent for cleaning purposes in most of the laboratories. Acetone is manufactured in different methods. Some of these methods include Dehydrogenation of isopropyl alcohol, the p-Cymene hydro peroxide process for p cresol and acetone, and Catalytic oxidation of isopropyl alcohol among others. Even though acetone is highly flammable, it facilitates in the storage and transportation of acetylene. Studies have proved that acetone does not pose a health risk if ingested in small amounts. Nevertheless, it might cause irritation of the throat if inhaled or irritation of the eyes if it is in contact with the eyes. Acetone is prevalent in water and soil. Nevertheless, it dissipates with time since microorganisms present in the water or soil consume it.
Reference List
Bahl, H, Andersch, W & Gottschalk, G 2000, ‘Continuous production of acetone and butanol by clostridium acetobutylicum in a two-stage phosphate limited chemostat’, European Journal of Applied Microbiology and Biotechnology, vol. 15 no. 4, pp. 201-205.
Behrend, N, Mahoney, B, Makarukha, I & Subramanian, S 2008, Uses of Acetone, University of Pennsylvania. Dept. of Chemical Engineering, Pennsylvania.
Darwent, B, Allard, M, Hartman, M & Lange, L 2000, ‘The Photolysis of Acetone’, Journal of Physical Chemistry, vol. 64 no. 12, pp.1820- 1847.
Lozano, A, Yip, B & Hanson, R 1992, ‘Acetone: A tracer for concentration measurements in gaseous flows by planar laser-induced fluorescence’, Experiments in Fluids, vol. 3 no. 6, pp. 369–376.
Wagner, I 1913, The condensation Products of Acetone, W.B. Burford, Indianapolis.
Young, J 2001, ‘Acetone’, Journal of Chemical Education, vol. 78 no. 9, pp. 1154-1175.