Experimental Requirements
- Two 100ml beakers, 10ml and 25ml Erlenmeyer flasks, and filter papers
- Acetone with a Molar mass of 58g/molecular and density of 0.79g/mol.
- Benzaldehyde with a molar mass of 106g/mol and a density of 1.04g/mol.
- 5g of sodium hydroxide
- Distilled water put in a wash bottle
- Ethanol
- Buncher funnel, filter paper, and a cork
Theoretical Calculations
The limiting reagent is usually insufficient for the reaction progress. Its insufficiency leads to a stop of the reaction when the other reactant remains active for reactions. To find the limiting reagent, we first calculate for the mass of dibenzalacetone produced by acetone, which is; 0.025×234=5.85g. On the other hand, the mass of dibenzalacetone produced by benzaldehyde is 0.05×234=11.7g. Acetone is the limiting reagent because it produces the least dibenzalacetone. Therefore, the moles of the limiting reagent is 0.025, and the mole of the product is 0.025. This is because their ratio is 1:1. The theoretical mass of the product would, therefore, be 0.025×234=5.85g.
Procedure
On my procedure, I mixed 0.0025 moles of acetone with 0.05 mole of benzaldehyde. I added a half of a mixture prepared by dissolving five grams of sodium hydroxide to 50ml water and 40ml ethanol. At this point, I observed a yellow precipitate. This implied that a new product was forming since the original solution was colorless. Fifteen minutes later, I added the aldehyde-ketone mixture that had remained. I rinsed the container to warrant that it was all transferred.
An hour later, I swirled the mixture frequently and used a Buchner funnel to collect the products. I reapplied the vacuum and repeated this step thrice to ensure that all sodium hydroxide traces were eliminated. I dried the crystal by pressing them using cork on the filter. I then used filter papers to remove most of the water. I preserved a small sample for the determination of the melting point. I then used about 10ml of ethanol to recrystallize the product for every four grams of dibenzalacetone. The reaction forming the yellow product was exothermic because it produced heat.
The melting point was 111.5 degrees Celsius. The mass of the product was four grams. This mass was less than the theoretical mass, which was expected to be 5.85 grams. This was due to the lost crystals during washing and re-crystallization. According to solubility chemistry, some of the crystals were dissolved at the early stages of the reaction. The product formed was an isomer of dibenzalacetone, which is (E, E)-1,5-Diphenyl-3-pentadienone.
Answers to the Experimental Questions
When one mole of aldehyde reacts with one mole of acetone in the presence of sodium hydroxide, it produces an organic substance known as benzal acetone. This product is also referred to as benzylideneacetone. The formation of this product relies on the mechanism applied. For this reason, I will describe the mechanism involved. Since acetone has pi hydrogen bonds on either side, the hydroxyl ions produced by sodium hydroxide react with one of the pi bonds to produce an enolate.
The enolate produced is nucleophilic. The aldehyde carbonyl, which is more electrophilic, reacts more with the enolate than the acetone to give an alkoxide. Water then protonates the alkoxide to produce β-hydroxy ketone, which is subjected to base-catalyzed dehydration to produce hydroxylate. The hydroxyl group in the hydroxylate is then removed to enable the formation of a double bond of benzal acetone.
We produced benzyl acetone using a base-catalyzed reaction of benzal acetone. In this case, we use a palladium catalyst or aluminum oxide. In this process, the alkene pi bond is removed to give benzyl acetone.
Equivalent proportions of the reagents ensure that the reaction takes place on only one side of the acetone producing benzal acetone. Otherwise, the use of excess benzaldehyde would produce dibenzalacetone.
In cases where excess benzaldehyde is used, traces of dibenzalacetone are expected as a result of disubstitution. If the amount of acetone used was in excess, it would also appear as an impurity. The other impurity is sodium hydroxide. In a bid to purify the product, we rinse it using water to remove sodium hydroxide. We also recrystallize it from ethanol for purity.
I observed that the melting point of the crude product is lower than that of the recrystallized product. This implies that the recrystallized product was pure, while the crude one is impure.
The geometric isomers of dibenzyalacetone were (E,E)-1,5-Diphynyl-3-pentadienone, (1E)-1,5-Diphenyl-1,4-pentadien-3-one and (1E,4E)-1,5-Diphenyl-1,4-pentedien-3-one.
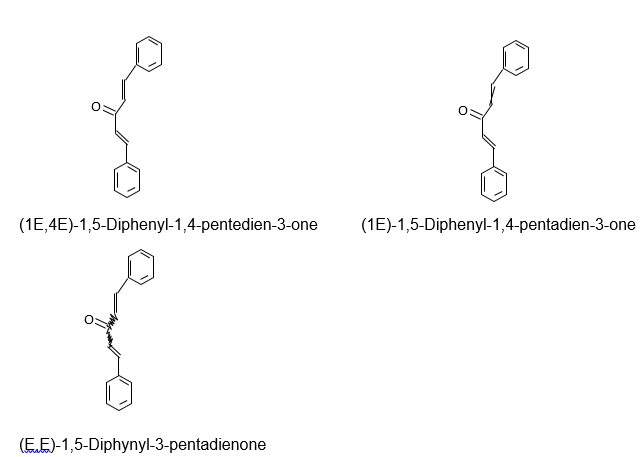
A pure isomer of dibenzalacetone with a melting point that incorporates 11.5 degrees Celsius is (E, E)-I.5-Diphenyl-3-pentadionone, whose melting points temperatures range from 110-112 degree Celsius.
I could also use the 1Hnmr spectrum of dibenzalacetone in the determination of the geometric isomers produced. This could be done by comparing the pairing constant between vinyl protons. The products in the experiment have two pairs of duplets around the fifth to seventh double bonds. These double bonds correspond to two sets of protons, which are not equivalent, placed on double bonds of the compound molecule. The protons contain a similar constant, K. However, the pairing constant between cis and trans-double bonds differ in a way that they cannot be assigned K as their constant.
An alteration could be made to the procedure of the experiment described above to produce benzal acetone. This could happen if I used equal moles of the reactants. If I used 0.05 moles of acetone and an equivalent amount of aldehyde, the resulting product when the appropriate time is considered would be benzal acetone. In order to produce benzalacetophenone, I could replace the acetone with acetophenone. In this case, acetophenone reacts with benzaldehyde in the presence of sodium hydroxide to produce benzalacetophenone.
The aldol condensation reaction involves the reaction between an aldehyde and a ketone using sodium hydroxide as the alkali. Acetone is deprotonated to form a nucleophilic enolate. The enolate formed is then subjected to a nucleophile. It is protonated to produce a neutral hydroxy ketone. Deprotonation takes place again to give rise to an enolate, after which the hydroxide is removed to generate an alkene pi bond. Contrary to the mechanism that was described in question one, the reaction that gives benzyl acetone requires that the reactants mole ratio be 1:1. It only happens when we use an equal volume of the reactants.
Equal moles of reactants warrant that the reaction does not take place on both sides of the ketone to produce dibenzalacetone. The point at which the crude product melts is lower than the pure one. Impurities lower the melting point. The isomer included is trans,trans-dibenzalacetone since the melting point of the product obtained has a melting point between 110-112 degrees Celsius. This melting point falls within the range of the isomer I stated. The product contains this single isomer since the rest has a melting point which is not within this range.