Waxes Production
Introduction
Waxes are malleable chemical compounds manufactured naturally or synthetically through chemical processes. Natural waxes are produced by both plants and animals during their physiological processes of life. In plants, the waxes are found on the cuticles, fruits, and leaves among other parts of the plants. In regard to animals and insects, they are found on the cuticles of the insects, fat deposits, and spermaceti among others.
The cuticles are formed from complex lipid chains that form a waterproof layer on plants and animals owing to their insolubility in water (Sturdy, 1972). On the other hand, oil refinery companies produce synthetic waxes that aim at producing massive volumes of wax for economic purposes. In this light, this research will consider the industrial production of waxes alongside their types, grades, and utilization. Characteristically, synthetic waxes are solids at ambient temperatures and melt above 45 degrees Celsius or equivalence of 133 Fahrenheit.
History of Waxes
The earliest form of wax was discovered over two thousand years ago. The wax was associated popularly with bee wax. The waxes existed in two color forms that included yellow and white brands. In fact, the early forms of waxes were produced by animals and plants. In this light, bees and spermaceti produced the animal wax that was the main source of wax apart from plants (Tolan, 2012). However, bees produced more wax than the spermaceti.
Consequently, human beings relied mainly on the bee for wax. During the 23rd AD to 79th AD, human beings discovered ways of purifying the yellow bee wax into white wax using salty water. The white wax was used in artistic work. For example, a Roman writer referred to as Pilny described this process of wax purification from yellow to the white wax brand. On the other hand, there is a limited amount of wax from plants for utilization on small scale.
These plants included the palm tree, shrubs, Japanese berry tree, and rice barns among others. The ideology of wax improved gradually and dispersed to the German empire leading to new discoveries concerning the use of wax. The increasing need forced people to find new ways of producing the present synthetic wax. Currently, synthetic waxes have dominated the wax industry and will form the basis of this paper.
Wax Separation Unit
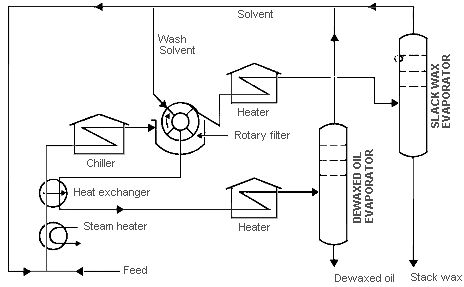
Wax is separated from oil through various methods that have been developed by oil engineers and technologists. They have done this in order to obtain high-quality products at the end of the process. The methods use a wax separating unit and involve three basic steps of chemical processes. In this light, this paper will consider the Solvent Dewaxing method that is highly used in the oil refineries company. It involves the three steps that include solvent mixture, precipitation, and solvent recovery.
Solvent Mixture
This step takes place in the steam heater. First, it should be noted that the wax and oil exist in solid form at low temperatures. They form a united chemical compound that cannot be separated in that form. Therefore, they must be dissolved in order to facilitate the process of separation. In this step, oil feedstock is mixed with suitable solvents that dissolve the oil and wax. In this light, toluene is used to dissolve the oil and maintain it in a liquid state rather than a solid-state. On the other hand, methyl ketone is used to dissolve the wax that is contained in the oil. The dissolved mixture moves through the heat exchange which cools the outgoing hot mixture and heats the incoming lube stock.
Precipitation
Oil and wax mixture proceeds to the chiller that facilitates the process of precipitation. In this case, the chiller lowers the temperature of the solution and triggers the Methyl Ketone to precipitate the wax into solid.
Solvent Recovery
This step takes place when the mixture moves into the rotary filter. The rotary filter separates the wax from the oil solution and methyl ketone. The wax proceeds into a heating chamber and later moves into the slack wax evaporator that produces the slack wax. On the other hand, the oil solution flows into the heat exchanger and the heating chamber. From the heating chamber, it flows into the Dewaxed oil evaporator that evaporates the oil and separates it from toluene through the fractionating column. Afterward, toluene is directed to the feedstock chamber to continue with the process. Therefore, the process has two end products that include the dewaxed oil and the stack wax
Hydro-finishing Unit
The slack wax has impurities that include Sulphur, metallic compounds, and nitrogen among other compounds. These impurities might corrode the surfaces of metals that come into contact with the wax. As a result, the wax must be purified to ensure that the wax is pure. The hydro finishing-unit is a unit that seeks to obtain type-I wax from stack wax and type-A wax. It achieves this purification by the process of hydrogenation which helps in saturating the hydrogen bond on the wax structure.
This lubricates the wax and produces a high-quality wax that is preferred to the original stack wax produced in the process of Dewaxing. The hydro-finishing unit has various chambers that carry out various roles in various processes in these chambers. In this case, the first chambers receive the slack wax and hydrogen gas which is the gas behind hydro-finishing.
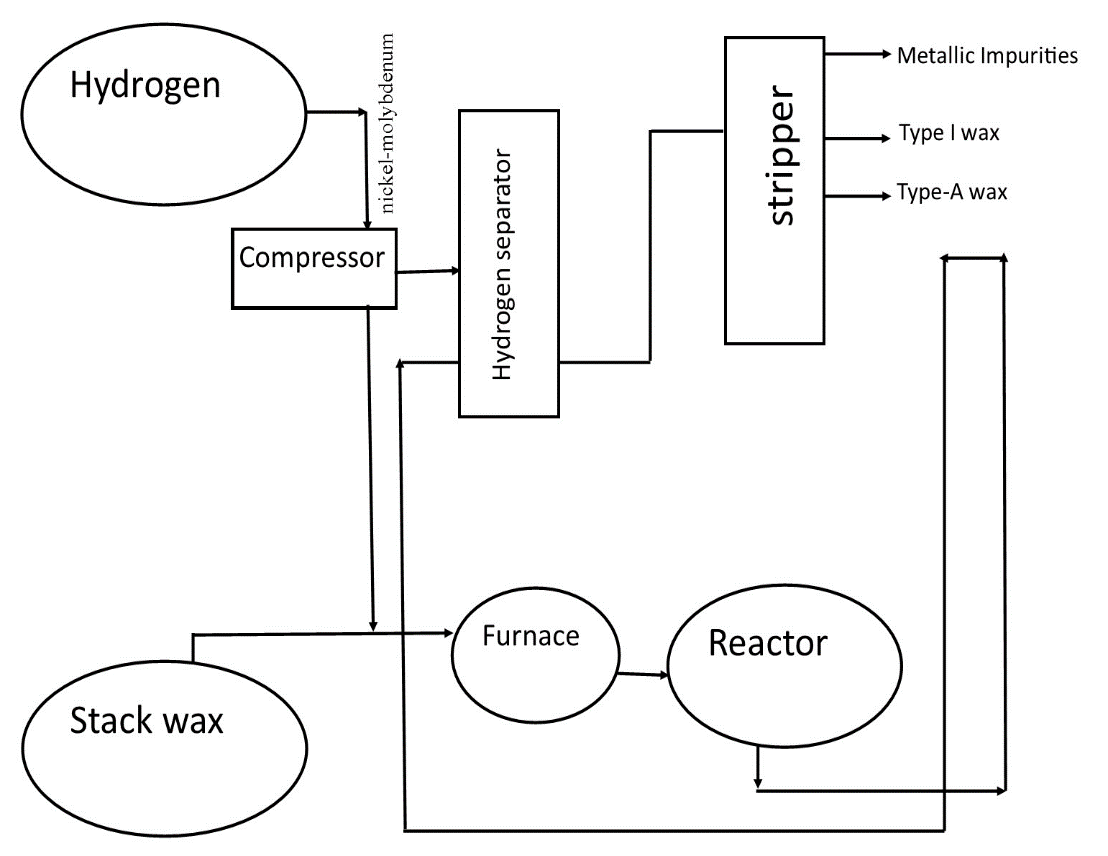
The mixture is reinforced with a nickel-molybdenum which is on alumina ensuring that it catalyzes the reactions taking place. The catalytic secret behind using a catalyst is that it reduces the activation energy that is required for starting up the reaction. Then the mixture enters into a compressor which increases the pressure to 2000 p.i.s.g. The compressed mixture moves into a heating chamber for thorough heating that increases the temperature up to 700 Fahrenheit.
The hot mixture enters the reactor and saturates the hydrogen bonds. The impurities are removed through hydrogenation. The latter moves to the hydrogen separator which separates the excess hydrogen from the products. The intermediate products move to the stripper and separate three components. These components include the impurities, type-A wax, and Type I wax. This wax is pure and free from impurities. It has more flexibility and has a high that protects it from oxidation by the atmospheric oxygen. This ensures that the wax can remain intact for a long time without breaking into parts. This is the diagram showing the basic structure of a hydro finishing process for the wax.
Types and Grades of Waxes Produced
There are various types and grades of waxes that are produced from petroleum. They have diverse and varying chemical and physical characteristics. Their classification, in accordance with chemical characteristics, is determined by the carbon compositions and presence of impurities among other factors. On the other hand, physical classifications are determined by the boiling points, physical appearance, color, and elasticity among others.
These classifications are combined to give various types and grades of the wax. In this light, it is crucial to understand that the grades appear in diverse natural circumstances or the manufacturing conditions. Some of these types include paraffin wax, refined wax, slack wax, scale wax, polyethylene wax, microcrystalline wax, petrolatum wax, beeswax, wax additives, recycled wax among other types. These types are categorized according to their existence mainly.
In regard to the grades of the microcrystalline, wax is classified as either hardening or laminating grades. The laminating grade melts at 145-175 F while the hardening grade melts at 175-200 Fahrenheit. The laminating grade can penetrate beyond 25while the hardening grade can penetrate below twenty-five. Other grades of waxes relate to the stage of the refinery. In this case, the paraffin waxes can be divided into three grades that include the slack wax, semi-refined wax, and scale refined wax. In this light, the three grades vary in purity. The slack wax is the most impure while the scale refined wax is the purest wax in that category.
In addition, there is a certain grade of waxes known as the food waxes. The food waxes are fairly elastic and sticky. This makes them suitable for repairing holes on corrugated iron sheets in order to prevent raindrops (Cheremisinoff & Rosenfeld, 2009). In regard to all categories of waxes that have been mentioned above, each category has various grades. This implies that the types are very many depending on the categories that exist. In addition, the current technology is developing the purification methods resulting in new grades.
Wax Specifications and Properties
The waxes that we have stated in the previous paragraph have diverse properties and specifications. They vary in their physical and chemical composition. Therefore, we seek to discuss a few of them in order to determine this variance and diversity. In this section, the paper will focus on three categories of waxes including microcrystalline waxes, paraffin waxes, and petrolatum waxes. In regard to the microcrystalline waxes, they are produced by removing oil from the petrolatum waxes.
These waxes have very tiny crystals that make it very cohesive, elastic, dense, and dark. They are produced by the refiners in a manner that meets the ASTM requirement. These requirements include a congealing point of A938, a color of D6045, needle penetration of about of D3121, and a viscosity of D445. The intermediate product is de-oiled to produce the microcrystalline waxes.
Secondly, this paper focuses on the paraffinic waxes that are produced from the oil in the oil refinery companies. They are white in color and are made of forty carbon atoms within their hydrocarbon chains. They are solids at room temperature, and they melt at 99F or equivalence of 37 degrees Celsius. The crystals are odorless, tasteless, and insoluble in water. This s because the crystals are non-polar as opposed to water which is polar in nature. On the other hand, they are soluble in inorganic solvents including benzene and ether. They are poor conductors and of electricity making them suitable for manufacturing insulators.
They are semisolid waxes that were used as ointments in the early days of developments. In the current world, they are used for producing skin products. One crucial product is the petroleum jelly that is used for milking animals. They are elastic and sticky in nature. Similar to paraffinic waxes, they are tasteless and insoluble in water.
Waxes Uses and Application
Waxes are used in various ways for the welfare of human beings and animals. They are very vital in the economic, social, and health arenas of a human being’s life. In a previous paragraph, we stated that the waxes were discovered over 2000 years ago in the Roman Empire in the form of beeswax. In this light, we appreciate that the waxes were used for animals, plants, and human beings. In regard to animals, the bees use wax for making shelters for their young ones.
They make a network of well-organized holes that facilitate the growth of their young ones. The insects use the waxes to make their bodies’ cuticles that protect them against pathogens. Similarly, the waxy cuticle of plants prevents the entry of pathogens into the plants and curb dehydration. In regard to human beings, there are various ways in which the waxes are used. In the olden days, the Greeks used waxes to seal envelopes for delivery. They used the waxes for arts by modeling houses and other things in the environment.
Waxes were used as healing ointments in China and other countries. In the modern world, they are used for making candles that are used in lighting. In fact, the churches have maintained the tradition of using candles as symbols of holiness. Some waxes, including the paraffinic waxes, are used for lubrication of moving parts owing to their elasticity and softness. In some instances, the waxes are used for binding documents together.
In this light, other waxes are used for repairing destroyed rooftops. They are used in the breweries industry for sealing wine bottles while the health industry uses them for dentistry. In fact, the list of applications is extremely lengthy. Specialists are discovering new uses of waxes on daily basis. In fact, the wax has become deficient and does not satisfy human needs.
Conclusion
This section has considered waxes in relation to the methods of production, grades of waxes, their properties, and significance in human lives. It has discussed the process of the refinery from the oil refinery companies alongside the steps that are involved in the purification of the wax. The section has managed to explain the history of waxes alongside their evolution in human history. In this light, it has touched on the most significant factors relating to the wax industry. Therefore, we can conclude that this section is all-inclusive and satisfactory. Now, we discuss the factors revolving around grease.
Grease Production
Introduction
Grease, which is famously known for lubrication, is a pseudo-plastic fluid that combines oil and soap. It possesses high viscosity that is reduced in the process of Thixotropy. In the process of Thixotropy, the grease undergoes a shearing process that reduces its viscosity significantly and increases its lubrication properties. In the process of making grease, oil is mixed with soap which acts as a thickener. It is crucial to notice that oil can be used as a lubricant. However, it lasts for a few days and disappears. On the other hand, the grease is thickened with soap. Therefore, it can last for a longer time than oil. The soaps manage to thicken the oil due to their ability of emulsifying other chemicals. They emulsify the oil to reduce the viscosity in a manner that needs the user to use a grease gun during application.
History of grease
Historians argue that the idea of making grease originated from the Egyptians and the Romans. This ideology was conceived by these territories in the nineteenth century. They suggest that the Egyptians and Romans combined Olive oil with lime, which occurred naturally on their territories, and allowed them to access the calcium carbonate for free. After mixing the chemicals, the chemicals experience the saponification of the chemical composition to produce calcium grease.
These chemical components were the triglyceride that is found in the olive oil. In 1958, oil was discovered in the United States of America triggering the discovery and the use of mineral oil lubricants. However, the lubricants could be used during the low temperatures only. Therefore, it was difficult to use them during the summers. This posed the main challenge to the utilization of the grease until the Second World War. In 1930, lithium thickeners were used to produce high-quality grease for lubrication. In the 1990s, the greases were replaced with improved greases comprising of propylene thickener. The propylene thickeners created greases that were better than the lithium ones. This development has persisted in the current world.
Grease Blending
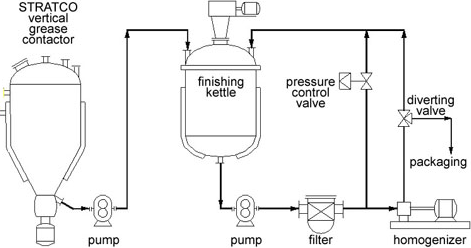
Grease blending is the process of mixing the original products in order to form grease. In this light, oil and soap are mixed in the contractor. At this point, the two ingredients wait for heating to raise the temperature and activate the process of a chemical reaction. When the reactants pass the impeller, their direction is changed resulting in turbulence. This turbulence causes a vigorous axial movement on the impeller.
The movement and introduction of heat lead to a rapid increase in temperature within a period of one hour. Moreover, the reaction that happens is exothermic. The exothermic reaction boosts the temperature to higher levels than the initial one. In addition, the mixture moves in two jackets of the reactor that retain a lot of heat in the system. These conditions increase the temperature to about 400 Fahrenheit. The pressure increases to a level of about 670 Kilo Pascal preventing the soap to produce foam in the system. The resultant products are pumped into the finishing kettle containing large assemblies that move slowly.
The slow-motion triggers the process of blending. In addition, it ensures that the intermediate products cool down due to cold water in the shell jacket. In this case, many additives are sensitive to temperature implying that they should not be put in the reactor (Rudnick, 2003). As a result, they should be introduced in the kettle when the products attain a reasonable temperature. Then, the manufacturers carry out an exercise called milling. Milling is a process that tests for homogeneity of the grease. Once it passes the homogeneity test, it is taken for packaging and storage.
Grease Types and Grades
The manufacturing industries produce various types of greases that differ in their chemical composition and the method of manufacturing them. In this case, they may differ due to the chemicals involved or conditions that are exposed to them. These conditions determine their viscosity, evaporation, and melting point. There are six broad categories of grease that arise from chemical composition categorization.
First, the grease might be made by mixing oil with solids. In this case, the greases are used for heavy machines operating for many hours. Secondly, grease might be made from a mixture of Asphaltic oils and light oils that have a lighter complexion. This grease forms a strong and protective layer on the moving parts reducing wear and tear. Thirdly, there are greases for extreme pressure that have additives. These additives improve the viscosity of the grease allowing it to withstand high pressure (Rivas, 2012). In addition, there are greases referred to as rock neck greases. It is a unique grease that lubricates plain bearings.
Lastly, there is soap that is thickened by greases containing soaps. In this light, the soaps are used to thicken the oil. The soaps that are used include calcium soaps, sodium soaps, and barium soaps. In addition, there are lithium soaps that are very popular and effective. During production, the manufacturers choose the soap they want to use. In this light, the soaps used for the process of manufacturing the greases determine their characteristics. Consequently, it determines the area of application and utilization. These greases, which are produced with respect to their metals, were the oldest greases that evolved gradually. The earliest soap was the calcium soap made from lime. On the other hand, the latest soap is lithium soap. It has been discovered lately due to the recent developments.
Grease Specifications
Grease specifications and characteristics vary according to their chemical composition. Their ability to withstand high temperatures and have long durability varies from one grease to another. In this case, we shall focus on the greases that have been thickened with soap. Their soap content is extremely high leading to the hardening of the lubricant at the high speed of the bearing. This makes them different from other greases.
Secondly, we should consider an aluminum greases. In this case, the greases are fabric and their dropping point are extremely high. They have the stability of shear, and their resistance to water is very high. Therefore, they protect the bearings and other moving parts from water and friction. The manufacturers recommend that the users apply this grease to the centralized systems that need the fabric element.
Moreover, lithium greases have the largest market share. They have moderate shearing ability, moderate dropping points, and good resistance to water. Also, we have polyuria greases in the market. They are manufactured for the high-temperature conditions during operations (Neale, 2001). They might have EP capability, but the EP capability might not be present. This relies on the choice of the manufacturer.
Lastly, the specialists have made synthetic greases. The synthetic greases have the best temperature and dropping point provisions. They withstand and cope up with high temperatures for the high speeding and heavily loaded vehicles and tractors. The grease work in a manner that allows the transfer of heat from the brakes to the bearings. This transfer reduces the amount of heat on the bearings. The reduction of heat on the bearings retains the lubricant for a long time.
Grease Applications
This paper has discussed the various greases that are available in the market and the manufacturing companies. Their applications vary according to the areas of application and utilization. These applications vary according to intensity, amount of grease, and parts of the application. For example, some greases are recommended for central systems while others are not recommended. Therefore, this paper will touch on some of the applications. First, greases are applied to machines and equipment during storage.
This ensures that the equipment or the machine does not rust during storage, especially when the equipment is stored for a long time (Han & Alzamora, 2011). Also, it ensures that the user will meet the machine in its original form. Therefore, grease is an essential tool for maintenance. Secondly, greases are used for machines that run in an internment motion. This helps them to avoid corroding their moving parts. As a result, they do not wear out during their operations.
Instead, they maintain their efficiency over a long time. Greases are used to lubricate machines that are potentially inaccessible. This inaccessibility makes it difficult to do frequent lubrication. Therefore, the users apply the grease to lubricate the machine ensuring that they are lubricated. Greases are used to lubricate machines that work in extreme conditions like high temperatures (Bunting, 1963). In this case, the use of oil would be insignificant and inappropriate.
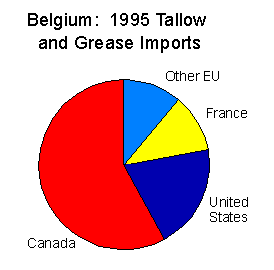
Therefore, using greases is more effective than oils. Lastly, greases are applied on the corroded parts of machines and equipment. In this case, the greases maintain a thick film on the surface of the corroded parts resulting in the extended life of the machine (Barnsby, 2003). The pie chart is an example of how countries import grease for utilization. It is very clear that Canada imports the highest scale of grease from Belgium.
This is an indication that the country has very many transportation logistics that require lubrication of facilities. As a result, they import a lot of greases that will be used for the lubrication of their facilities. The second-largest importer of grease is the United State of America. However, Canada imports more grease than the USA. This is because the USA has oil deposits that enable them to make grease. On the other hand, Canada has little oil deposits that force them to rely on imports. France is the third main importer of the grease while other European Union companies are the fourth. This shows that grease is a major component of a country’s economy. It cannot be ignored in the budget of the country. This is because it is a component of maintenance.
Conclusion
This section has touched on greases and the factors that revolve around them. In this light, it has discussed the history, types, specifications, and applications of the greases in various fields of the operation. In this light, it has presented the process of manufacturing greases and blending. In addition, the paper has shown the various grades of grease that are produced in the process of manufacturing. Also, it has shown the specification of the greases. Lastly, it has shown the exports of various companies and how they compare with each other. Therefore, it has presented worthy research.
References
Barnsby, R. (2003). Life ratings for modern rolling bearings: a design guide for the application of international standard ISO 281/2. New York: ASME International.
Bunting, K. R. (1963). DEVELOPMENT OF GREASE LUBRICANTS FOR HIGH TEMPERATURE BALL AND ROLLER BEARINGS OF ELECTRICAL EQUIPMENT. Ft. Belvoir: Defense Technical Information Center.
Cheremisinoff, N. P., & Rosenfeld, P. E. (2009). Handbook of Pollution Prevention and Cleaner Production Best Practices in the Petroleum Industry. Burlington: Elsevier Science.
Han, J., & Alzamora, D. A. (2011). Geo-Frontiers 2011 advances in geotechnical engineering. Reston, VA: American Society of Civil Engineers.
Neale, M. J. (2001). Lubrication and reliability handbook. Boston: Butterworth-Heinemann.
Rivas, E. (2012). Unit operations of particulate solids: theory and practice. Boca Raton, FL: CRC Press.
Rudnick, L. R. (2003). Lubricant additives chemistry and applications. New York: M. Dekker.
Sturdy, H. A. (1972). Wax. Ilford: Central Association of Bee-keepers.
Tolan, K. (2012). The Wax. Alexandria, VA: Alexander Street Press.