Aims
The objective of this experiment was to determine the enthalpy of an acid-base reaction. As such, a resulting value would be compared to a theoretical value to determine the accuracy of the experiment.
Method
In this experiment, a 50 ml volume of 1M HCL was put into a polystyrene container, stirred and, the temperatures were taken after every 20 seconds for three minutes. Of note, caution was taken to minimize the time of contact with the container as this would raise the temperatures of the container hence giving a false impression of the actual temperatures. Using the same equipment but washed and dried, the same procedure was performed for a 50 ml 1M NaOH. The two solutions were then quickly mixed, stirred, and the temperatures were taken after every 20 seconds for a duration of 3 minutes. A graph of temperature against time was then plotted and as such the temperature change was established.
Results
From the results, the change in temperature (∆T) obtained was 6.5 K. The values of constants given were: the specific heat capacity (C) of a 0.5M NaCl (4.01JK-1g-1) and, the density of 0.5M NaCl at 298K (1.04gcm-1). From the data, the calculated enthalpy (QD) obtained was -54.215KJmol-1(refer to the appendix). On the other hand, a theoretical value is given as -57.3KJmol-1.
Discussion
In this experiment, the main objective was to determine the heat of neutralization of a reaction between HCL and NaOH. By definition, the heat of neutralization results when a molecule of water is formed from hydrogen and hydroxyl ions present in an acid and a base respectively. With regards to this experiment, the two components represent a reaction between a monoprotic acid and a monobasic base. Hence, apart from the formation of a salt molecule in an ensuing reaction a molecule of water is expected as an end product. Significantly, the constituents of the mixture represent a reaction between a strong base and a strong acid. To this end, complete dissociation is expected by the end of the reaction (Lemke, p. 43).
Referring to the preceding discussion, on reacting a monobasic base and a monoprotic acid a molecule of water and salt is expected. As such, the reaction below happens:
NaOH(aq) + HCL(aq) → NaCl(aq) + H2O(l)
Separately focusing on the individual components gives us a clue of what transpires in an aqueous solution of both strong acids and bases. Since they dissociate completely the below reactions takes place:
HCL (aq) → H+ (aq) + Cl–(aq)
NaOH(aq) → Na+(aq) + OH–(aq)
From the equations above it is evident that in an aqueous solution both components detach completely resulting in a high concentration of both hydrogen and hydroxyl ions. Consequently, for this reaction, a lot of heat is expected. Significantly, in this reaction, the active components bringing about the enthalpy change are H+ and OH– ions. Sodium and chloride ions represent spectator elements in the reaction.
Literature value for these kinds of reactions gives an approximate figure of -57KJmol-1. However, this value increases in that order depending on the degree of hydrogen ions present in strong acid. For instance, for a reaction involving a diprotic acid (e.g. sulphuric acid) and sodium hydroxide, two molecules of water are formed hence the value obtained is -114KJmol-1. The sign preceding the value is significant in giving a clue of what kind of reaction takes place; either exothermic or endothermic. For an exothermic reaction, heat is released to the environment while the opposite is true for endothermic reactions. As such, when holding a container having an exothermic reaction your hand feels warm. The vice versa is true for endothermic reactions. When calculating, a negative value depicts an exothermic reaction while a positive value represents an endothermic reaction. With respect to this experiment, the reaction represents an exothermic reaction. This is schematically represented by the figure below:
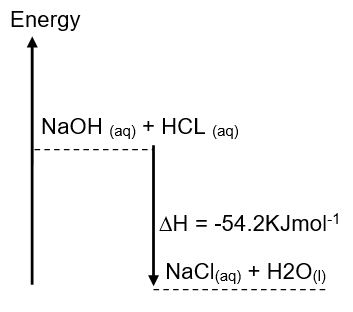
As regards this experiment, the result obtained was below the expected. The discrepancy in enthalpy was 3.2 KJmol-1. This can be attributed to several factors that might have occurred in the course of the experiment. To this end, factors touching on insulation against heat loss might have had a greater effect on the results obtained (Crawford, p. 34). Moreover, in the calculation there is a need to factor in the quantity of heat absorbed by the container otherwise if neglected then the value obtained will always be below the expected.
Summary
The objective of this experiment was to determine the enthalpy of an acid-base reaction with the main focus centered on HCL versus NaOH reaction. As such, the experimental value for enthalpy was negative (-54.2 KJmol-1). This showed that the reaction is exothermic. However, this value differed from the theoretical owing to experimental errors. Of note, this reaction depicts a neutralization reaction between a strong acid and a strong base hence; the components dissociate completely resulting in relatively high enthalpies (Guggenheim, p. 23).
References
Crawford, F.H 1963, Heat, Thermodynamics, and Statistical Physics, Rupert Hart-Davis, London.
Guggenheim, E.A 1993, Modern Thermodynamics by the Methods of J.W. Gibbs, Methuen, London.
Lemke, T. L 2003, Review of Organic Functional Groups: Introduction to Medicinal Organic Chemistry, Lippincott Williams & Wilkins, New York.
Smith, J. M 2005, Introduction to Chemical Engineering Thermodynamics, New York, Macmillan.
Appendix
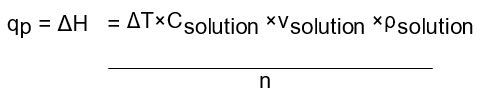
Where: ∆H is the enthalpy change
n is the amount of reaction mixture
∆T is the change in temperature
C is the specific heat capacity of the solution
Ρ is the density of the solution
v is the volume of the solution
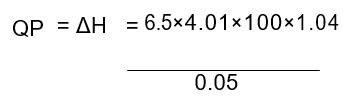
=54215.2 = -54.215 KJ mol–1.
Literature Values = -57 KJmol-1