Introduction
Laboratory technologists’ duties do not only consist of performing analyses. These specialists are also responsible for the accurate collection, transportation, and reception of specimens. Al-Rahba has a number of policies regulating the mentioned procedures.
Specimen Collection
The collection of specimens is a significant element of the pre-analytical phase that may impact the validity of results received within analytical and post-analytical phases. Specimen collection at Al-Rahba is carried out by qualified laboratory specialists or nurses. There are regulations and policies for each type of specimen (1). For instance, collecting blood specimens is possible at the Northern Laboratory reception while body fluids cannot be collected there. Collecting blood specimen may take place in the wards, the accident and emergency department, or in outpatient clinics. Body fluids are collected by patients and stored in special containers. If a patient is unable to collect urine fluids, special types of collection are employed, such as catheterization, suprapubic aspiration, and ureter catheterization. Laboratory technologists explain all procedures and their specifications to the patients (2). Blood specimen collection incorporates the following:
- specimen collection by STAT and routine analysis;
- providing the security of the sample while carrying it to the laboratory;
- specimen transportation by porters or phlebotomists;
- guaranteeing safety for the patient and the specialists collecting the specimen;
- making sure that all materials and equipment used for collecting blood specimens are not expired.
Transportation of Specimen
At Al-Rahba, the transportation of specimens is performed by a porter. If a porter is not available, a phlebotomist or a nurse should carry the specimen to the laboratory as soon as possible. Containers should have secured lids that prevent leakage. Body fluid samples are transported in biohazardous plastic bags. All specimens should be carried in an upright position. Labels should be located on test containers rather than on biohazardous bags since the latter are discarded after transportation.
Porters should carry the samples to an accessioned area of the laboratory. They must not leave the samples unattended. If there is no one in the reception area, a porter should press the bell and wait until someone accepts the specimens.
There are some exceptions concerning transportation:
- heparinized syringes, blood gases analysis, and capillary tubes are immersed in ice and carried immediately to the laboratory;
- Vitamin D, Ammonia, PTA, BNP, and Lactate specimens should also be delivered immediately and transported in ice;
- special coagulation tests should be spun twice during 30 minutes, and the plasma should be separated and frozen at -80˚C;
- specimens of quanteferon should be incubated at 37˚C.
Specimen Reception
There are several general precautions concerning the reception of specimens in Al-Rahba (3):
- laboratory employees should wear laboratory coats;
- technologists should wear gloves and change them whenever there is a possibility of contamination;
- lab workers should use facial protection devices;
- laboratory surface must be kept clean;
- red biohazard containers should be used for disposal of contaminated items;
- sharp objects must be disposed of in sharps containers.
The procedure of specimen reception takes place when a phlebotomist makes rounds in the unit. After collecting samples, the phlebotomist gives them to a porter or a nurse, who carries them to the laboratory. It is necessary to make sure that the information on the sample label matched the data on the manual requisition form through the hospital information system (HIS). All specimens should be logged into the Center system.
Criteria for Patient Identification for Request and Samples
Patient Identification is the ability to properly identify and keep a single patient record for every patient. HIS contains patient demographic data and any other relevant information. Each level of analytic process presupposes different criteria for patient identification. During the pre-analytic process, the phlebotomist has to introduce himself/herself to the patient and ask for at least two identifiers (full name, medical record number, or the date of birth). The procedure of patient identification is reflected in the following flowchart:
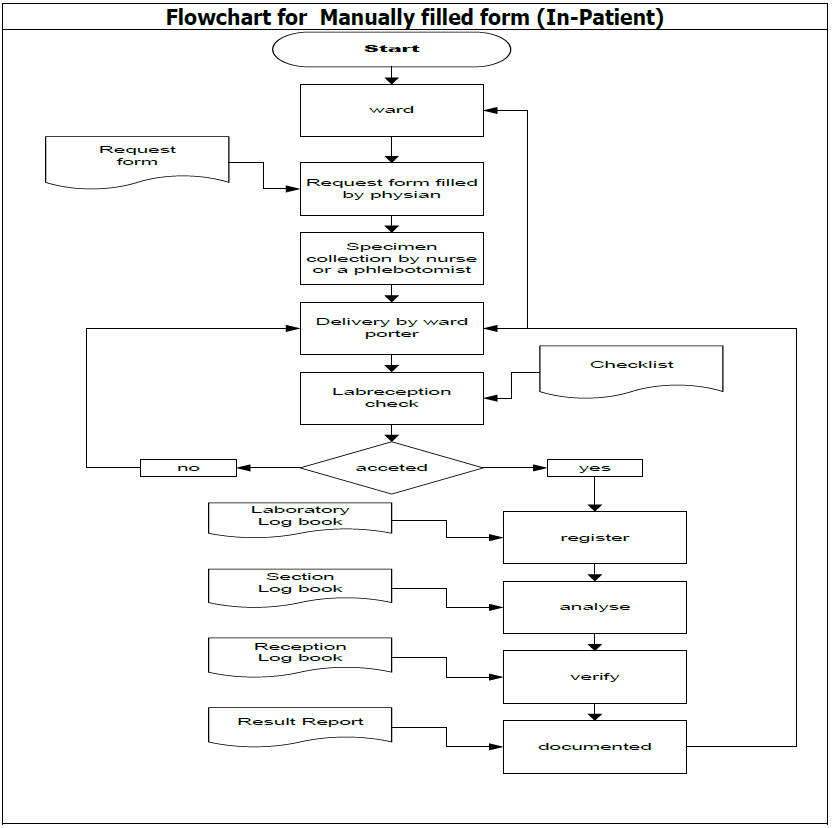
Criteria for samples are the following:
- the cup or position number should be related to the patient’s name when the sample is transferred to equipment;
- if samples are poured into a secondary tube, each aliquot should be labeled by two identifiers;
- it is preferable to use printed labels.
Examples of Potential Problems, Ways of Resolving Them, and Potential Consequences of Errors
During collecting and transportation of specimens, it is necessary to follow the requirements concerning temperatures (4). An example of a problem is when urine is kept at room temperature for more than one hour. The potential consequence of such a problem is that the analysis will not be precise since bacteria may utilize urine glucose. To prevent such an error, it is necessary to use chemical preservatives if there has been no possibility to put urine samples in the refrigerator.
Another potential problem is associated with specimen reception. A laboratory employee may neglect the requirements concerning patient identification, which may lead to misplacing the results. To prevent such a situation, all employees should be thorough when collecting patient data and make sure to collect a minimum of two identifiers.
Conclusion
In order to provide the best quality laboratory results, technologists have to ensure that specimen collection, transportation, and reception are performed in accordance with the regulations. Al-Rahba hospital takes care of its patients and employees by arranging the most suitable conditions for collecting and storing samples. When problems arise, the ways of resolving them are suggested by the employees, as well as precautious measures to be taken in the future.
References
- Health Authority of Abu Dhabi. HAAD clinical laboratory standards. Abu-Dhabi: HAAD; 2011.
- Joint Commission International Accreditation Standards for Hospitals. 6th ed. Oak Brook: Joint Commission International; 2017.
- Hill RH, Finster DC. Laboratory safety for chemistry students. 2nd ed. Hoboken: Wiley; 2016.
- College of American Pathologists. All common checklist: CAP accreditation program. Northfield: College of American Pathologists; 2015.