Introduction
Stable radicals are molecules that have odd electrons and actively seek to enter into reactions. These molecules live long enough compared to free radicals, which react instantly. Therefore, stable radicals can be studied and even isolated using traditional chemical methods. Methods for isolating stable radicals have been developed by scientists since the late 19th century, and this area of chemical science is still relevant due to its applied value. For example, modern chemistry uses antioxidants to stabilize free radicals in cosmetics and other products. The main purpose of isolating stable radicals is to obtain new chemical reactions for study and control. This paper will examine the literature on stable radicals, their synthesis, and the functions of the main families of stable radicals.
Background on Stable Radicals
Further, the main properties of stable radicals will be presented, and families of stable radicals and their synthesis will be described. Ashenhurst (2021) defines stable radicals as “neutral, electron-deficient chemical compounds with a partially filled orbital; they are highly reactive intermediates in organic chemistry” (para. 1). The main distinguishing characteristic of stable radicals is their high reactivity due to the presence of one or more unpaired electrons. Chemists have also developed more stable types of stable radical families with high kinetic stability (Ratera et al., 2021). In such families, stabilization is performed by changing the charge and the odd electron and visually such molecules look identical. During the substitution, the actual average distribution of electrons within the molecule is considered to be intermediate between the two configurations of a radical with a charge and a radical with an odd electron.
All free and stable radicals have the ability to magnetic resonance, and, according to quantum physics, the process of resonation or delocalization greatly increases the stability of the material. In the case of stable radicals, an increase in stability determines the very probability of their existence (Ratera et al., 2021). Remarkably, free radicals also become more resistant due to delocalization, but for a shorter time. Radicals are subvalent molecules that can easily participate in covalent bonding processes, such as connecting to dimers or going through hydrogen abstraction, recombination, or disproportionation, to meet valency (Chen et al., 2020; Tang et al., 2021). This rationally explains organic radicals’ inherent thermodynamic and kinetic instability. However, traditional tools such as normal spectroscopy and magnetic resonance studies can properly analyze the structures and properties of many persistent and stable radicals. While their open-shell shapes remain, some of them are stable enough to be separated as pure compounds or crystals.
Verdazyl Radicals
Verdazyl radicals are a family of highly stable radicals that have been isolated through the insertion of a 6-membered ring containing four nitrogen atoms. This artificial compound was experimentally discovered in 1963 (Shimizu & Osuka, 2018). Verdazyl radicals are high-modulus chemicals, their key feature, which makes them of practical interest, is the ability to a wide range of substitution sequences. Their structural variety and stability have been exploited in the disciplines of materials, inorganic, polymer, and physical chemistry. Nevertheless, their deliberate usage as starting materials for organic chemistry has yet to be explored.
In recent years, the isolation of verdazyl radicals has been carried out at the George Laboratory, which was the first to report the synthesis. In 2008, George’s scientific group discovered by discovering a disproportionation reaction involving 6-oxoverdazyl radicals. Remarkably, this reaction leads to the formation of azomethine imines capable of 1,3-dipolar cycloaddition, which allowed scientists to use verdazyl radicals as substrates for organic synthesis (Shimizu & Osuka, 2018). The next discovery made it possible to discover that the utility of verdazyl radicals in the synthesis is due to cycloadducts generating azomethinimine derivatives. But, more importantly, radical reactions involved an increasing number of N-heterocycles generated by cycloadducts via rearrangement reactions. Equally important, as part of the discovery, George’s group discovered that a subclass of verdazyl radicals, the triphenylverdazyl radicals, exhibit particular reactions with alkynes. These reactions proceeded by direct addition of radicals and rearrangement to form isoquinolines.
As a result of preliminary research, scientists have developed two new strategies for the synthesis of stable radicals. First, by expanding the available alkyl substituents in the N1 and N5 positions of the 6-oxoverdazyl radicals. And secondly, through the development of asymmetric examples of 6-oxoverdazyl radicals with non-identical N1 and N5 alkyl substituents (Headley, 2020). The effect of various alkyl substituents on the disproportionation reaction of 6-oxoverdazyls has aroused particular interest among scientists. Kuhn and Trischmann’s original paper on verdazyl radical-6 from 1963 provides the basis for one of the most common triaryl verdazyl radicals with sp3 centers at the C6 position (Headley, 2020). Within this reaction, methylation or alkylation of formazan with any other primary alkylating agent causes subsequent oxidation to form a verdazyl radical.
The main disadvantage of employing this approach to make triaryl verdazyl radicals is the C3 aryl group’s limited tolerance for electron-withdrawing groups. Recently, the synthesis of a 6 verdazylium cation ring starting with formazan and an aldehyde in acidic circumstances was reported (Headley, 2020). The verdazylium ion was reduced to an anion in base by ascorbic acid, which is then oxidized in air to form the verdazyl radical. This synthesis is more versatile for C3 aryl groups and can be utilized with substituted aldehydes to introduce C6 substituents. The formaldehyde-based synthesis can be used in the Mannich reaction, allowing amine substituents to be introduced at the C6 position.
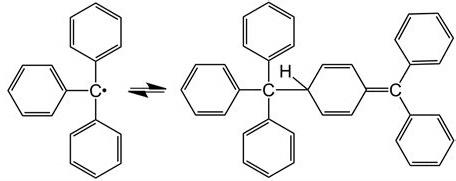
Triphenylmethyl Radicals
Triphenylmethyl is the first stable free radical artificially developed by chemists. It was isolated by Moses Homberg in 1900 (Shimizu & Osuka, 2018). Triphenylmethyl has three substituents instead of four, its lone electron is represented by a dot, and it is trivalent. Triphenylmethyl free radicals show stability only in certain organic solvents. Stability is rapidly destroyed by irreversible processes in the presence of air, water, or strong acids (Headley, 2021). Free radicals are formed upon breaking the nitrogen–nitrogen bond in aromatic hydrazines with the general R2N–NR2 structure. Another possible way of formation is by breaking the central nitrogen–nitrogen bond in aromatic tetrazines with the general structure R2N–RN–NR–NR2.
Other known free radicals with a similar formation process have odd electrons on oxygen, such as the 2,4,6-tri-tert-butylphenoxine radical (Shimizu & Osuka, 2018). Then, the reaction of benzophenone with another stable radical ion results in the formation of a ketyl metal. Therefore, when treated with sodium metal, chemists obtain a paint material (C6H5)2CO. The reaction described occurs because when sodium reacts with complex aromatic hydrocarbons such as naphthalene, a side reaction is the appearance of brightly colored radical ions. Another class of stable organic free radicals is the > NO group. An example of reactions with these radicals is the production of diphenylnitrogen oxide (C6H5)2NO by oxidation of diphenylhydroxylamine (C6H5)2NOH (Shimizu & Osuka, 2018). The presence of stable free radicals depends on the structural properties of the molecules. One interesting property is demonstrated by semiquinone radical ion IV. The top oxygen atom of this radical has a negative charge, and the bottom one contains an odd electron.
Polychlorinated Trityl Radicals
Of particular note is the process of stabilization of polychlorinated trityl radicals, also known as polychlorotriphenylmethyl (PTM) radicals. Stabilization occurs when significant steric hindrance caused by chlorine atoms in the ortho orientation protects their single unpaired electron located on the central carbon atom. Attractive properties of PTM include rich electrochemistry and special visual properties. Equally important, PTMs have inherent magnetic rotation due to the unpaired electron, accompanied by absorption and emission (Ratera et al., 2021). Interestingly, these PTM functions can be fine-tuned by creating a large collection of PTM derivatives. Recent discoveries regarding PTMs include the use of these radicals in molecular electronic compounds or switches (Ratera et al., 2021). Switches are used as building blocks for the production of magnetic networks, in bioapplications, and for the creation of optoelectronic devices and materials.
The properties of radicals are reflected in their ability to synthesize with other elements. PTMs are formed from three chlorinated phenyl rings linked to a central carbon atom via sp2 hybridization (Ratera et al., 2021). These radicals remain chemically and thermally stable due to the steric barrier at the central carbon atom, created by the ortho-chlorine atoms on the phenyl rings, which retain these radicals. As a result, the PTM-containing molecule assumes a stable propeller shape due to the interchlorosteric tension. Therefore, PTM radicals become resistant to oxygen and a wide range of harsh chemicals, and they are even stable in solid form when exposed to light. PTM radicals withstand conventional synthetic methods, which have allowed the development of a wide range of materials with special magnetic properties, such as high spin polyradicals. Notably, PTM derivatives also exhibit magnetic characteristics, allowing further experimentation.
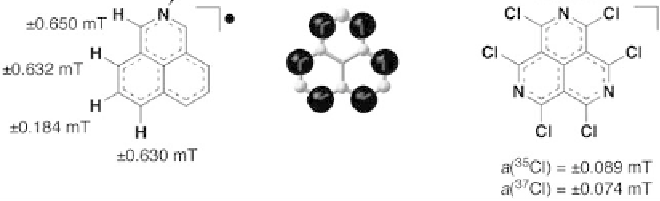
Carbon-Centered Radicals: Phenalenyls
Phenalenyl-based stable radicals have easily controlled electrical characteristics associated with the substitution of a heteroatom. Smeu, Monti, and McGrath (2021) explain that this family of stable radicals has controllable spin and charge-transfer capabilities in unimolecular compounds. The process takes place using the non-equilibrium Green’s function, which uses the theory of the density function in the calculations. Therefore, stable radicals based on phenalenyl create the prerequisites for experiments due to powerful spin filtering and unusually high conductivity.
Carbon-centered radicals are formed in atom transfer reactions: as a rule, in such reactions, a halogen atom is transferred, or, sometimes, hydrogen atoms. During synthesis or conversion, absolute rate constants are established for the formation of carbon-centered radicals when halogenated chemicals react with Bu3Sn (Smeu, Monti, & McGrath, 2021). The atom transfer reactions (Me3Si)3Si and Et3Si have a comparable set of rate constants. During synthesis, one atom in the substrate must be more reactive than any other atom or group. If this condition is met, during the reaction, the radical is formed selectively, through atom transfer. For example, in a typical propagation step pair, an iodine atom is transferred from a carbohydrate to a tin-centered radical, and this pair produces a carbohydrate radical.
Carbons have a fundamental structure that allows the creation of carbon-centered and oxygen-centered (alkoxy) radicals. Hydrogen abstraction and radical fragmentation are two processes that define oxygen center radicals (Smeu, Monti, & McGrath, 2021). A radical centered on a carbon atom is formed when an oxygen-centered radical is cleaved. Therefore, fragments of the alkoxy radical form a radical with a carbon center and an open ring. Fragmentation can occur without detachment of a hydrogen atom since there is no effective hydrogen donor in such a reaction mixture.
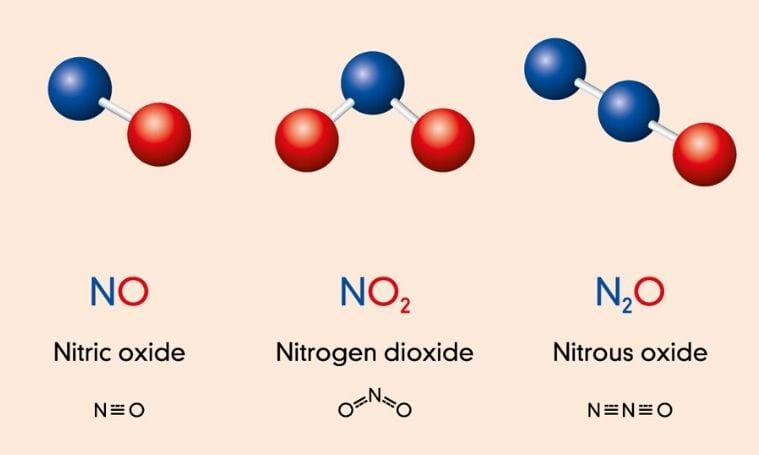
Nitrogen Oxides
Nitrogen oxides are used to produce fertilizers and other chemical products. Nitric oxide can be created using the performance of a milliscale sliding arc reactor, under atmospheric pressure conditions. Patil et al. (2018) investigated the frequency, pulse width, amplitude, and feed rate of a sliding arc reactor. The experiments were carried out at a rate of 1 L/min in the sliding arc discharge mode (Patil et al., 2018). The highest concentration of NOx was found to be 1% for energy consumption of 10 kWh/kg NOx. The scientists observed an increase in specific energy input and NOx concentration as frequency, pulse width, and amplitude increased.
DPPH
The hydrazyl stable free radical (DPPH) is another significant family of stable radicals. They are open-shell compounds and their chemistry has been continuously developed since the discovery of the stable free radical 2,2-diphenyl-1-(2,4,6-trinitrophenyl)hydrazyl (DPPH) in 1922 (Ionita, 2021). Experiments with DPPH have led to many discoveries in the theory and practice of free radicals. The authors have been actively exploring the chemistry and physics of stable free radicals, including organic reactions, since 1968 (Ionita, 2021). However, in recent studies, scientists have not paid enough attention to the free radicals of hydrazyl and amine.
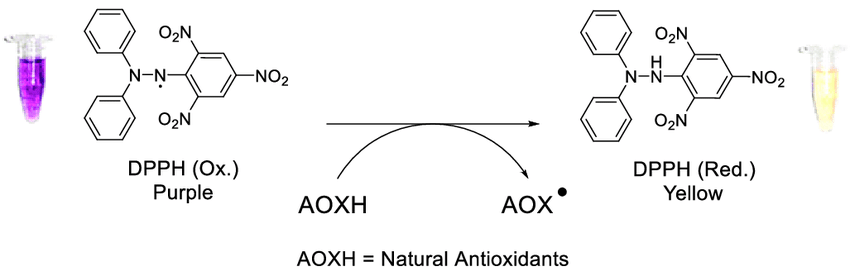
Interestingly, since the discovery of 2,2-diphenyl-1-(2,4,6)-trinitrophenyl, scientists have focused on the chemical and physical properties of this free radical, including participation in the formation of stable hydrazyl (DPPH). More recently, scientists have used the acronym DPPH as a synonym for the term “antioxidant analysis” (Ionita, 2021). Notably, DPPH makes it possible to demonstrate the stability of hydrazyl free radicals. The resonant structures of DPPH clearly show a push-pull action. This happens because the pyric fragment plays the role of the electron-withdrawing part of the molecule, and the diphenylamine fragment plays the role of the electron-donating part (Ionita, 2021). Equally important, DPPH has a larger dipole moment (4.88 D) than DPPH-H (3.59 D) and this makes the polar resonance structures important (Ionita, 2021). At the same time, the X-ray structure indicates that the NN bond has a higher bond order. Due to its high stability, DPPH is widely used as a standard in electron spin resonance (ESR) spectroscopy.
Compared to 2,2-diphenyl-1-(2,4-dinitrophenyl)hydrazyl, the DPPH free radical makes it easy to establish stability (Ionita, 2021). There is a difference in stability between the two 2,4-dinitro and 2,6-dinitro isomers 1 and 2 since the most important nitro groups are in the ortho position relative to the nitrogen atom. Isomer 2 (2,6-dinitro) can exist in both solution and solid form, while isomer 1 (2,4-dinitro) can only exist in solution and has a half-life of 90 hours (Ionita, 2021). Remarkably, most DPPH free radicals are stable at position 6 with any other group.
Phenoxyl Radical Complexes
Scientists are actively studying the interaction between redox-active transition metal ions and redox-active ligands. Galactose oxidase (GO) uses two one-electron cofactors, a copper atom, and a cysteine-modified tyrosine residue, to catalyze the aerobic oxidation of primary alcohols to aldehydes. This compound is a typical example of phenoxyl radical complexes (Oshita & Shimazaki, 2022). To study the electronic structure of the oxidized form of the enzyme (GO(ox)) analogs of small molecules, including complexes of metal-salen phenoxyl radicals, are considered. These one-electron oxidized complexes are mixed-valence compounds (Oshita & Shimazaki, 2022). MOs with phenolate and phenoxyl bind by mixing with metal d-orbitals and act as redox centers.
Recently, theoretical, spectroscopic, and electrochemical approaches were used to study the electronic distribution in these unusual kinds of electrons. This approach provided valuable information about the electronic structure of GO(ox) (Oshita & Shimazaki, 2022). Equally important, over the past ten to fifteen years, scientists have paid great attention to the study of metal-coordinated phenoxyl radicals because of their biological significance. Metalloenzymes such as class I ribonucleotide reductase, prostaglandin-endoperoxide synthase, glyoxal oxidase, and galactose oxidase (GO) have a tyrosine moiety, which is an interesting example of metal-ligand cooperation in a redox process.
Conclusion
Thus, this paper discusses stable free radicals and their families. This work deals with DPPH, nitrogen oxides, phenoxyl radical complexes, nitroxide radicals, carbon-centered radicals, verdazyl radicals, triphenylmethyl radicals, and polychlorinated trityl radicals. Of particular interest are the properties of these families in synthesis processes and methods for their stabilization. Stable radicals were first discovered in the early 20th century, and for more than 100 years, chemists have been deriving new compounds experimentally. Today, stable radicals are of interest for many applied and fundamental reasons. One of the main reasons is that these odd-electron molecules live long enough to be explored and put to practical use.
References
Ashenhurst, J. (2021). 3 factors that stabilize free radicals. Web.
Chen, X., Zhao, W., Baryshnikov, G., Steigerwald, M., Gu, J., & Zhou, Y. et al. (2020). Engineering stable radicals using photochromic triggers. Nature Communications, 11(1), 10-13.
Headley, A. (2020). Organic chemistry: Concepts and applications. Wiley.
Ionita, P. (2021). The Chemistry of DPPH. Free Radical and Congeners. International Journal of Molecular Sciences, 22(4), 1545.
Oshita, H., & Shimazaki, Y. (2022). Stacking Interaction of Metal Phenoxyl Radical Complexes. Molecules, 27(3), 1135.
Patil, B. S., Peeters, F. J. J., van Rooij, G. J., Medrano, J. A., Gallucci, F., Lang, J., & Hessel, V. (2018). Plasma assisted nitrogen oxide production from the air: Using pulsed powered gliding arc reactor for a containerized plant. AIChE Journal, 64(2), 526-537.
Ratera, I., Vidal-Gancedo, J., Maspoch, D., Bromley, S., Crivillers, N., & Mas-Torrent, M. (2021). Perspectives for polychlorinated trityl radicals. Journal of Materials Chemistry C, 9(33), 10610-10623.
Shimizu, D., & Osuka, A. (2018). Porphyrinoids as a platform of stable radicals. Chemical Science, 9(6), 1408-1423.
Smeu, M., Monti, O., & McGrath, D. (2021). Phenalenyls as tunable excellent molecular conductors and switchable spin filters. Physical Chemistry Chemical Physics, 23(42), 24106-24110.
Tang, B., Zhao, J., Xu, J., & Zhang, X. (2020). Tuning the stability of organic radicals: from covalent approaches to non-covalent approaches. Chemical Science, 11(5), 1192-1204.