Introduction
The amorphous solid forms indistinguishable, disorganized, and irregular organization of constituent particles. They lack the typical three-dimensional long-range organization of crystal structures, thus, amorphous solids have a much more randomized arrangement of molecules. The term amorphous originated from the Greek word ‘omorphe’, which means without shape (Gado, 2018). They also demonstrate short-range order across several molecular dimensions and have physical properties that are significantly different from that of their crystallized counterparts. Amorphous solids are similar to liquids throughout as they lack an ordered structure or three-dimensional arrangements of atoms or electrons. The solid to liquid conversion occurs across a wide temperature range since these substances do not have a definite melting point. Amorphous substances have a physical property that is usually isotropic in nature, meaning that they are unaffected by measurement directions that have the same magnitude throughout all directions. The purpose of this report is to describe the history, structure, properties, and applications in real life of amorphous solids.
History of Amorphous Solids
Amorphous solids are defined by a hard structure despite having chaotic groupings of atoms or bigger particles. Some examples of amorphous solids are clay, plastic, and glass. One of the first researches of porous crystalline bodies were conducted by W.L. Bragg and H. Bragg (Day, Drake, Zhou, & Ryder, 2012). In addition “some of the earliest structural studies of porous materials were conducted in 1930 by future two-time Nobel Prize winner Linus Pauling” (Day, Drake, Zhou, & Ryder, 2012, 2). Over time, “new experimental and computational techniques have enabled recent advances, improving the understanding and control of materials at the molecular level” (Day, Drake, Zhou, & Ryder, 2012, 3). However, the fact that far too many events are fundamentally unaffected by the lack of a periodic lattice, has necessitated a rethinking of the prevailing theoretical framework.
The paradox has compelled chemists to learn more about solids, which has led to a greater understanding of chemical principles and the relationship between bonding and bands. When combined with the unexpected occurrences brought on by disorder, the stage is now set for intense activity in amorphous structures. Bernall’s theoretical work mostly on the structure of liquids, together with Scott’s experimental random which involved packing of spheres, sparked interest in the arrangement of atoms in amorphous solids in the 1960s (Powell, 2019). During the recent decade, the investigation into atomic arrangements of amorphous materials has been divided into two streams: firstly, more refined and complicated research of sphere as well as molecule packing, and secondly, atomic simulations through molecular dynamics.
Structure of Amorphous Solids
The fundamental atoms of matter within a solid are ordered randomly, meaning that the positions of elementary particles are really not fixed rather vary from one amorphous solid to another. Due to the general random pattern of atoms or molecules within the amorphous lattice, amorphous materials have no defined structure or geometry (Nicolas et al., 2019). Moreover, amorphous solids contain short-range order. Since they lack crystalline structure as shown in figure 1 and have flow properties, amorphous solids are sometimes referred to as pseudo-solids as well as super-cooled liquids.
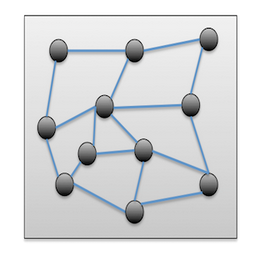
Properties of Amorphous Solids
Amorphous solids display the following qualities, they possess a short-range order, have no sharp melting point, are pseudo solids in nature, are isotropic, have indefinite heat of fusion, and show rough and irregular surfaces when cut into two. In addition, they have an irregular atomic structure. The molecules of an amorphous solid have a short-range order of action (Singh, 2020). They may, however, have little areas of the ordered plan. Crystallites are transparent fragments of a usually amorphous substance. An example is at Curie temperature which has local ordering also referred to as a short-range order. When a moment points in one direction, its neighbors are more likely to do so as well. This trend persists over several lattice sites, never over vast distances.
Wide Range of Melting Temperatures
Amorphous solids do not have a distinct melting point, but they do melt at a wide range of temperatures. For example, when glass is heated, it begins to mellow and then melts across a temperature range. As a result, glass can be molded or blown into a variety of shapes (Lalena et al., 2020). The warmth of fusion is not present in amorphous solids. When an amorphous solid is heated and then gradually cooled through toughening, it becomes translucent at a certain temperature. As a result of some crystallization, glass objects from antiquity appear smooth.
Electrical Properties
When an electrical current is applied across an amorphous solid, it offers high resistance to the flow of the current. Insulation resistance is defined as the property of a substance that prevents the flow of electrical energy. It needs to be as high as feasible for a material to be called a good insulator. There are two forms of insulation resistance: volumetric resistance and surface area resistance. Volumetric resistance refers to the resistance provided to the current flowing through a material. Surface area resistance refers to the resistance provided to the current flowing across the material’s surface area. Whenever temperature changes, moisture presence, the voltage supplied, and aging are all factors that affect the insulating resistance properties of insulators.
Dielectric Strength is defined as the lowest voltage that will cause an insulating material’s insulating characteristics to be destroyed when voltage is applied. It is also known as the highest electrical potential gradient that a material structure can withstand before rupturing or losing its dielectric characteristics. The insulating material thickness is measured in volts or kilovolts per unit thickness. The circumstances have a significant impact on this value.
The operating circumstances of the material have a significant impact on its electrical resistance value. Environmental humidity and operating temperature are two factors that influence dielectric strength. Plastics and other amorphous solids, for example, plastics offer very high resistance to the flow of electricity and are thus called good electrical insulators. As a result, they are employed to line electric wires for protection and electronic equipment.
Amorphous Solids are Isotropic
Amorphous solids possess isotropic properties, in other words, they have consistent qualities across all directions. For instance, an amorphous solid’s properties such as thermal conductivity, coefficient of thermal expansion, and refractive index all have the same value regardless of which way the properties are tested. This property is important and it means amorphization can be achieved by rapidly cooling when in the molten state. This can also be achieved through the freezing of the solid’s vapor (Goldsmith et al., 2017). The particles are unable to form a crystalline pattern as a result of drastic changes in temperature. If quartz, the crystalline material of SiO2, is heated to melt and then quickly cooled, it forms an amorphous solid called silica glass. This substance has the same chemical structure as quartz but loses atomic orderliness. Through the same process whenever thin layers of melted metal are rapidly cooled, they create amorphous metal alloys. The metallic glasses that form are stronger, more malleable, and more chemically inert than crystalline combinations of the same structure.
Difference between Crystalline and Amorphous Solids
The main feature differentiating crystalline and amorphous solid is the length scale across which related atoms are arranged translational symmetrical. This arrangement can either be periodic in nature or through the long-range organization. Crystalline solids fall into two broad categories: single crystals and polycrystals. Amorphous materials and liquids have no long-range order, while single crystals have unlimited periodicity and polycrystals have localized periodicity.
Ideally, a single crystal has an arrangement of atoms that repeats itself across its entire volume on a regular basis. Translation symmetry connects every atom in the configuration to just about every other corresponding atom inside the structure, also at indefinite length scales (DeGuili, 2018). Boulder salts, quartz crystals, as well as calcites are examples of single crystals. Many separate grains or crystallites make up a polycrystalline solid or polycrystal. Every grain could be regarded as a single crystal having a long-range structure in its atomic arrangement. There is no link between neighboring grains inside an isotropic polycrystalline material. As a result, there is also no periodicity along with a polycrystalline material on a broad sufficient length range. Rocks, salts, or sands are just a few examples.
On the other hand, because amorphous materials, such as window glass, lack long-range organization, therefore lose translation symmetry. The organization of an amorphous solid (or even a liquid) is not totally random; the spacing connecting atoms is highly defined and equivalent to that of a crystal as shown in figure 2. And that is the reason liquids and crystals possess identical densities although liquids contain short-range order, which keeps atom distances fixed, while crystals exhibit a long-range type of arrangement (Disha, 2020). Glasses, candy resins, and plastics are just a few examples of amorphous solids. The key differences between crystalline and amorphous solids are as summarized in table 1 below.
Table 1: the difference between crystalline and amorphous solids (Lalena et al., 2020).

Examples of Amorphous Solids
Amorphous solids typically are made up of both natural and human-made substances. Thus, amorphous solids are found in all solid material subsets. Amorphous materials include glassware, ceramics, gels, resins, polymers, plastics, cream, and waxes. Glass is frequently used in the manufacturing of packaging items for instance kitchen jars, beauty boxes, and beverages bottles, dinnerware (utensils), and building construction materials such as windows, lights, and shelves, among other applications. The most common real-life applications of amorphous solids include transparent glasses, the manufacturing of polymeric structural materials, amorphous semiconductors used in electronics, and magnetic amorphous solids used in making storage devices.
Transparent Glasses
Because of historic building utilization of amorphous solids like glass and window glass are so well-known, and the words are frequently used in every household. Transparent glasses such as oxide glasses are great in permitting light inside, but they are also excellent at preventing cold. Therefore, they are efficient thermal insulators hence they are widely used in building energy management for energy conservation and energy efficiency as shown in figure 3. Amorphous solids are also used in the manufacturing of advanced technological applications that contribute to modern progress. One such application is the use of pure glass in signal transmission in the telecommunications sector. The transparency property of the ultra-pure glasses designed for fiber-optic telecommunications is so high that light can flow through one kilometer of glass yet still sustain 95% of its initial intensity at specific wavelengths.

Amorphous solids mostly in the type of glass fibers commonly used in transmitting optical signals are currently used exactly for what copper wires are famous for. That is the transmission of electricity; they can also be made to obtain higher efficiencies than copper. In addition, glass fibers are currently used in transporting telephone signals throughout the world. The mode of operation is that a semiconductor material is attached to one end of the glass fibred to convert the sound waves to light signals. The light signals are then conveyed as a sequence of light pulses through a lengthy piece of cable. On arrival at the point of use, it is turned into electrical waves and subsequently into sound for the end-user to understand.
Polymeric Structural Materials
Polystyrene and other amorphous solids are significant lightweight structural elements that are widely used in the automobile, aerospace, and construction sectors. Plastic molded products made of these materials are also common in everyday life. In terms of quantity, the amount of polymer materials manufactured each year is greater than the amount of steel produced. Plastics is one of the most prominent thermoplastic polymers that softens and flows fluidly when melted to near glass transformation temperature, allowing it to be produced at incredible speeds and on a widespread basis in the creation of molded items as shown in figure 4.

Amorphous Semiconductors
Thin films of amorphous superconductors are useful in applications that need huge areas of electrically active materials as shown in figure 5. One large-scale electronic application of amorphous semiconductors is xerography. This is a type of electrical imaging technology that underpins plain-paper copiers. The thin-film, substantial are photo transmitting element at the center of the xerographic process is made of amorphous selenium (Se) or amorphous arsenic selenide (As2Se3). The photosensor is subjected to a photograph of the paper to be reproduced, which is an insulating material in the dim light yet transmits electricity whenever lighted.
Day after day, the xerographic process produces well over 5 billion photocopies in workplaces, library resources, and institutions throughout the globe. The same technology is used in the manufacturing of laser printers and solar cells to produce electricity (Singh, 2020). In laser printers, the amorphous photoconductor material is subjected to an electronically controlled on-and-off laser light that is sequentially placed over the photoconductor substrate. Solar cells with amorphous silicon thin films are also used to generate power for handheld calculators. This essential amorphous semiconductor is also utilized as a photosensitive receptor in xerographic copiers, as well as a photodetector in facsimile machines.

Magnetic Amorphous Solids
Magnetic features of amorphous solids, for example, magnetic glasses, are useful in technological designs that require magnetism. Magnetic glasses are physically hard materials with a magnetic softness that allows them to be magnetized easily by tiny magnetic fields. Moreover, they possess greater electrical impedance than typical crystalline magnetic substances. This is because they have a disorganized atomic-scale structure (Gado, 2018). Magnetic glasses are ideal materials that find application in the manufacture of magnetic cores that form the crucial part of electric power transformers.
They are preferred because of their ease of fabrication, possess magnetic softness properties, and offers high resistance to the flow of electricity. Excellent electrical resistance is a critical attribute in this application since it reduces energy loss and decreases undesirable electric eddy currents. Thus, strips of amorphous magnetic glasses are employed as a transformer-core coating in electricity power conversion applications because of these favorable properties as shown in figure 6. Magnetic glass thin films are utilized in a variety of additional applications, including magnetic storage media for sound and visual digital recording, and also magnetic disk capture heads.

Conclusion
Amorphous solids have an atomic structure that is disorganized and are characterized by qualities such as short-range order, lack of sharp melting point, isotropic, indefinite heat of fusion, and show rough and irregular surfaces when cut into two. They also do not have a specific melting point but melt at a wide range of temperatures. Amorphous solids are the most common types of solids and include materials such as glassware, ceramics, gels, resins, polymers, plastics, cream, and waxes. Therefore, amorphous solid find application in all spheres of human life. For example, glass is frequently used in the manufacturing of items such as kitchen utensils, beverages bottles, dinnerware, and building construction materials such as windows, lights, and shelves.
References
Day, G. S., Drake, H. F., Zhou, H.-C., & Ryder, M. R. (2021). Evolution of porous materials from ancient remedies to modern frameworks. Communications Chemistry, 4(1), p. 1–4.
DeGuili, E. (2018). Field theory for amorphous solids.Physical Review Letters, 121, 118001. Web.
Disha, E. (2020). Foundation course in chemistry with case study approach for JEE/ NEET/ olympiad class 9. 5thed. Disha Publications.
Gado, E. (2018). Constructing a theory for amorphous solids.Physics, 11, 88. Web.
Goldsmith, B. R., Peters, B., Johnson, J. K., Gates, B. C., & Scott, S. L. (2017). Beyond ordered materials: Understanding catalytic sites on amorphous solids.ACS Catalysis, 7(11), 7543-7557. Web.
Lalena, J. N., Cleary, D. A, Olivier B. M., & Duparc, H. (2020). Principles of inorganic materials design. John Wiley & Sons.
Nicolas, A., Ferrero, E. E., Martens, K., & Barrat, J. L. (2018). Deformation and flow of amorphous solids: Insights from elastoplastic models.Reviews of Modern Physics, 90(4), 045006. Web.
Singh, J. (2020). Optical properties of materials and their applications. John Wiley & Sons.