One of the most important reactions of organic synthesis is the Diels-Alder process, in which an alkadiene with multiple conjugated bonds forms a substituted cyclohexene derivative when interacting with an alkene. This reaction is essential for biochemical studies because the formation of cyclohexenes by the [4+2]-cycloaddition mechanism is not uncommon in the secondary metabolism of the plant and animal organisms.
In addition, this process is also essential for fine synthesis because it is a one-step reaction, and the binding of the first and fourth carbons of the cis-diene component to the alkene is highly accurate for obtaining a specific product. In the present experiment, a semi-microscale study of the IR spectra of the recrystallized product and raw materials is carried out to confirm the reaction between anthracene and maleic anhydride. The fundamental hypothesis is the assumption that the reaction, according to the Diels-Alder mechanism, leads to the formation of an entirely new substance that has different properties from the reagents.
On the other hand, the unique [4+2]-cycloaddition will result in some of the functional groups within the formed molecule repeating the same elements in the raw material, which means that IR spectrometry should give not wholly different data. It is expected that the spectrogram of the product of the reaction under study will not only have peaks similar to those of the feedstock but also new peaks unique to the interaction of anthracene and maleic anhydride.
Equipment
- 10-mL graduated cylinder
- 13 x100-mm test tube
- 2 melting point capillary tubes
- 2 utility clamps
- 25-mL round-bottom flask
- 3-mL product vial
- hot plate
- microspatula
- support stand
- watch glass
- 250-mL beaker*
- boiling chip
- Büchner funnel, with adapter
- condenser, with tubing
- electric flask heater
- 50-mL Erlenmeyer flask
- 125-mL filter flask, with vacuum tubing
- filter paper
- glass stirring rod
Reagents
Table 1. Reagents used in the experiment, with descriptive characterization.
Procedure and Description
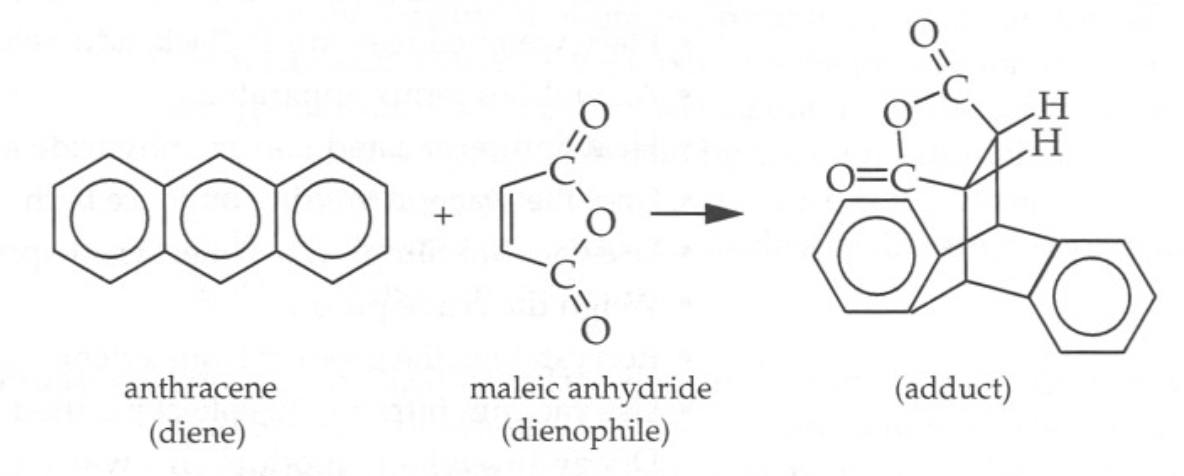
All steps of the study were performed precisely as suggested by Wade & Whitman (1998) unless otherwise indicated. The general procedure of the entire experiment combined four sequential steps. The first of these involved the interaction between anthracene and maleic anhydride, as shown in Figure 1. It can be seen that anthracene acts as a diene hydrocarbon, while the role of the alkene is played by the anhydride. The product of their interaction — 9,10-dihydroanthracene-9,10-aß-succinic anhydride — is also shown in Figure 1 on the right side. The ratio of a diene to alkene 1:0.5 or 0.5 g of anthracene to 0.25 g of maleic anhydride is used for the synthesis: both substances are dissolved in 6 mL of xylene.
Using the reflux device shown in Figure 2, the reaction product is crystallized by initial constant heating of the mixture for half an hour and then cooling in an ice bath. As a result of the cooling, the liquid substance transitions to a solid phase state, which allows the organic synthesis product to be collected in a proper and usable form. The precipitate from this step was subjected to filtration on a Buechner funnel.
The second step of the procedure was the purification of the reaction product by recrystallization. For this purpose, in a heated medium of hot xylene, the entire mass of the product was transferred to the liquid phase: the amount of xylene was gradually added to the boiling mixture until the grains of the solid were no longer distinguishable. Subsequent cooling of the solution again caused crystallization, which was enhanced by scratching the bottom of the Erlenmeyer flask.
At the same step, preparation of the collected and recrystallized substance for further study was made. For this purpose, the solid was collected by vacuum filtration and then washed several times with ice-cold xylene. The pure product, devoid of impurities, was spread evenly in a single layer on thin glass. The sample was left to dry for half an hour, after which a visual check of readiness was made: this included moisture, evenness, and dryness. Before the next step, the dry product was weighed on an analytical scale, and its weight was recorded in a test log.
The third step of the experiment was to compare the melting point of the recrystallized product with the starting substances that had been used as raw materials. It was expected that the product should have a different IR spectrogram from the feedstock, which would fully justify the expectation in the atomic transformation by the [4+2]-cycloaddition mechanism. A potassium bromide tablet, standard for studies of this kind, was used as a mulch. IR spectra were taken for both the recrystallized product and for anthracene and anhydride. The fourth step was to clean the laboratory equipment and properly dispose of the experimental waste.
Data Analysis
The IR spectrometry results obtained in step three of the procedure are used as tools for data analysis. The absorption bands and peaks at characteristic wavelengths for the product, for anthracene, and for anhydride are compared. The results of this comparison are logged in detail, from which the conclusions of the entire study are subsequently formed.
Reference
Wade, L., & Whitman, J. (1998). The Diels-Alder reaction of anthracene with maleic anhydride. Modular Laboratory Program in Chemistry.