A Balancing Act
- Physiological processes of organisms’ operation in environment.
- Relative concentrations of fluids and solutes maintained at optimal levels.
- Osmoregulation controls solute concentrations and balances the gain and loss of water.
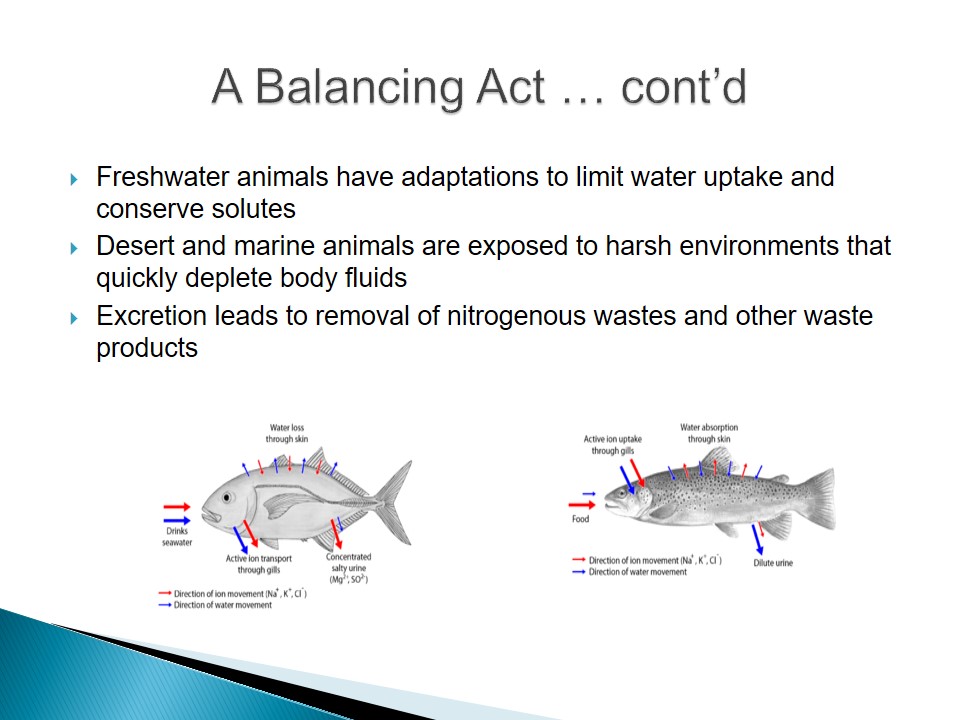
- Freshwater animals have adaptations to limit water uptake and conserve solutes.
- Desert and marine animals are exposed to harsh environments that quickly deplete body fluids.
- Excretion leads to removal of nitrogenous wastes and other waste products.

Osmoregulation
- Active regulation of the osmotic pressure (osmoregulation).
- Maintain the homeostasis of water content.
- Prevents too diluted or too concentrated body fluids.
- Sustain right fluid concentration.
- Osmotic pressure is required.
- Osmosis is observed.
- Higher osmotic pressure causes more water movement.
- Aquatic and terrestrial organisms must sustain the right solute concentration.
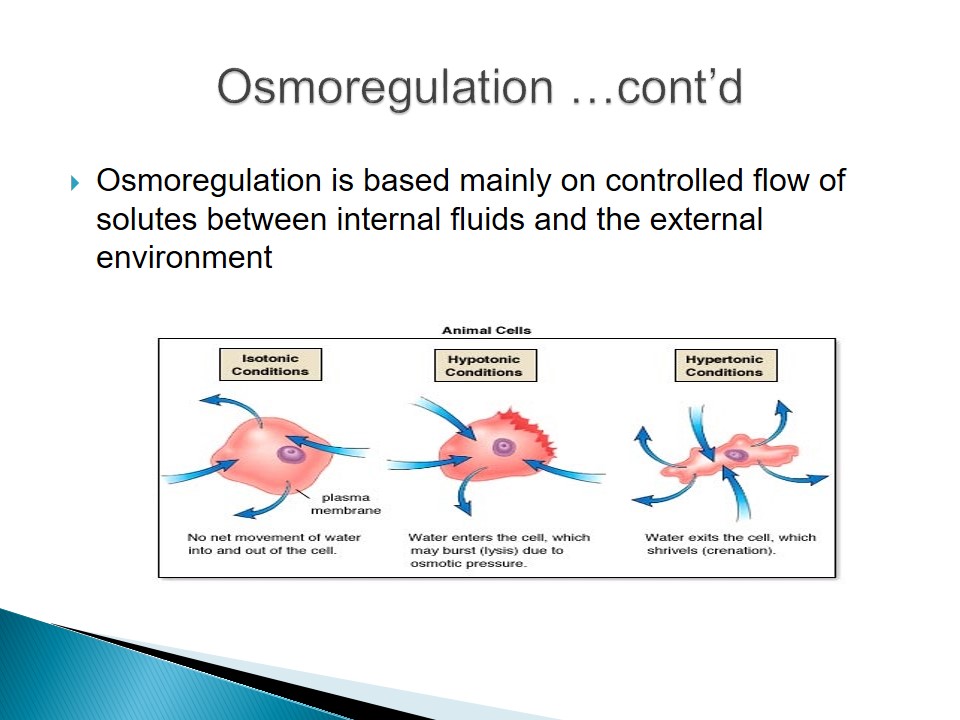
- Osmoregulation is based mainly on controlled flow of solutes between internal fluids and the external environment.
Osmosis and Osmolarity
- Cells need stability between osmotic gain and water loss.
- Osmolarity refers to solute concentration.
- Osmolarity controls the flow of water across a selective permeable membrane.
- Isoosmotic solutions have equal movement of water particles.
- Differences in osmolarity lead to water movement from the hypoosmotic to the hyperosmotic solution.
Osmotic Challenges
- Osmoconformers (mainly consisting of certain marine animals) are isoosmotic with their environment and thus do not control osmolarity.
- Osmoregulators consume energy to regulate water absorption and loss in hyperosmotic or hypoosmotic environment.
- Many animals are stenohaline, i.e., they cannot withstand significant fluctuations in external osmolarity.
- Euryhaline organisms are able to survive major fluctuations in external osmolarity.


Osmoregulation & Excretion
- Kidney function:
- Keep a balance of body salts at optimal:
- A challenge with terrestrial organisms is water conservation;
- There is a need to replace lost water;
- Animals can conserve water;
- Adaptation mechanisms are necessary for terrestrial organisms such as producing concentrated urine, being active at night and drinking among others.
- Remove metabolic wastes;
- The main challenge is nitrogenous wastes from protein;
- Getting rid of nitrogenous wastes as:
- Ammonia;
- Urea;
- Uric acid.
- Keep a balance of body salts at optimal:

Excretory Processes
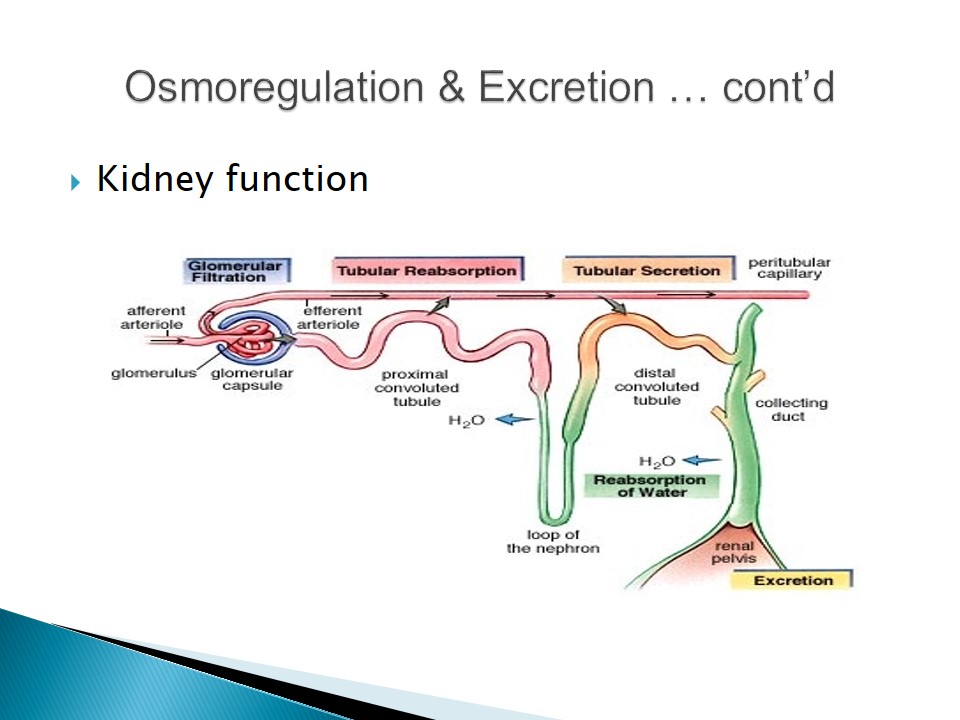
- Excretory systems release urine by refining filtrate fluids obtained from body fluids.
- Major roles of most excretory systems:
- Filtration: pressure-filtering of body fluids;
- Reabsorption: reabsorbing useful solutes;
- Secretion: releasing toxins and other solutes from the body fluids to the filtrate;
- Excretion: removing the filtrate from the system (Campbell & Reece, 2007).
Hormonal Relations
Antidiuretic Hormone:
- Nervous and hormonal control of water and salt reabsorption in the kidneys control osmolarity of the urine (Agrawal, Agarwal, Joshi, & Ghosh, 2008).
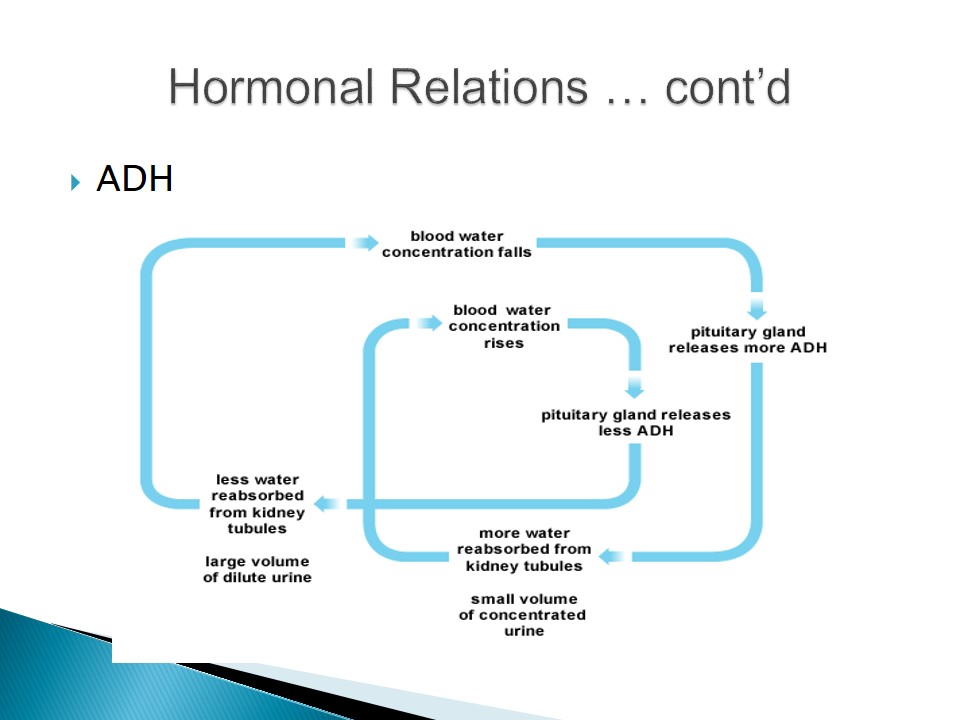
- Antidiuretic hormone (ADH) is responsible for increased water reabsorption in the distal tubules and collecting ducts of the kidney.
- An increase in osmolarity activates the release of ADH to assist in water conservation.
- Impair in ADH production causes serious water loss and results in diabetes insipidus.
- Alcohol is a diuretic and prevents the release of ADH.
Pathophysiology
- Disorders of salt and water balance may occur (Evbuomwan, 2013).
- There are clinical and biochemical changes resulting from this disorder of salt and water balance.
- Such changes result in adverse osmoregulation function.
- Disturbances of osmoregulation in humans are shown through a change in serum sodium concentrations (Sinke & Deen, 2011).
- Serum sodium concentrations cause hypernatremia or hyponatremia.
- Hypernatremia is an increased serum sodium concentration above 145 mM.
- Hyponatremia (serum sodium concentration below 136 mM).

Findings
- A disturbance in the water balance is an abnormality shown in the serum sodium concentration and causes hypernatremia or hyponatremia.
- Resultant morbidity could be insignificant, serious, or even life-threatening.
- Research focuses on the management of hypernatremia, emphasizing a quantitative approach to the correction of the fluid imbalance (Adrogué & Madias, 2000).
- Patient-centered approach is required to determine underlying causes and effective interventions (Giddens, 2013).
Pharmacology Treatment Regimen
- The maintenance of enough body fluid volume and effective supply of this fluid between the body cells is a vital part of homeostasis (Lord, 1999).
- Osmosis determines water movement.
- Osmometry is an important part of the management of many patients (Lord, 1999).
- Osmotic process has a significant role in some therapeutic activities of drugs and its strength needs to be determined in fluids administered to patients.
- Effective treatment of hypernatremia needs a two-pronged approach (Adrogué & Madias, 2000):
- Addressing the underlying cause.
- Correcting the prevailing hypertonicity.


Conclusion
- Osmoregulation controls solute concentrations and balances the gain and loss of water.
- Organisms must adapt to their various environments to survive.
- In human, disturbances of osmoregulation in humans are shown through a change in serum sodium concentrations, which are responsible for hypernatremia or hyponatremia.
- Treatments should patient-centered and aim to:
- Addressing the underlying cause.
- Correcting the prevailing hypertonicity.
References
Adrogué, H. J., & Madias, N. E. (2000). Hypernatremia. New England Journal of Medicine, 342, 1493-1499. Web.
Agrawal, V., Agarwal, M., Joshi, S. R., & Ghosh, A. (2008). Hyponatremia and Hypernatremia : Disorders of Water Balance. Web.
Campbell, N. A., & Reece, J. B. (2007). Biology (8th ed.). San Francisco: Benjamin Cummings.
Evbuomwan, I. (2013). The role of osmoregulation in the pathophysiology and management of Severe Ovarian Hyperstimulation Syndrome. Human Fertility, 16(3), 162-167. Web.
Giddens, J. (2013). Concepts for Nursing Practice. St. Louis, MO: Mosby.
Lord, R. C. (1999). Osmosis, osmometry, and osmoregulation. Postgraduate Medical Journal, 75(880), 67-73. Web.
Sinke, A. P., & Deen, P. M. (2011). The physiological implication of novel proteins in systemic osmoregulation. The FASEB Journal, 25(10), 3279-3289. Web.