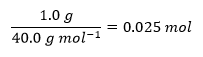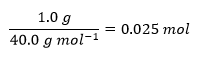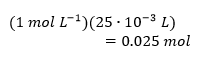Summary
The colorimetric method of analysis is used to determine the heat released during a chemical or physical process: the heat released in such reactions is transferred to the water by the change in temperature, of which it is possible to determine the thermodynamic parameter of the reaction itself. In the present laboratory work, the calorimetric method is used to determine the heat in three consecutive exothermic processes, which determine the reaction of neutralization of sodium hydroxide in hydrochloric acid. The first step characterizes the dissociation of the solid alkali in water, resulting in the formation of a sodium cation and a hydroxyl group anion.
The second stage determines the interaction of solid alkali with hydrochloric acid, and the third stage determines the interaction of the already soluble form of sodium hydroxide with acid. Since each step generates some heat (in kJ), it is necessary to use Hesse’s law to determine the total heat of the reaction. Specifically, Hess’ law states that in complex processes, the heat effect of a reaction is not conditioned by the intermediate states and reaction pathway and is determined only by the states of the starting substances and products.
In other words, in applying Hess’ law to three consecutive reactions, there is no need to detail all the calculations, and the final heat of the neutralization reaction can be calculated only from the heat of each of the three processes. Thus, the purpose of this laboratory work is to calculate the heat of the total reaction of the dissolution of sodium hydroxide and subsequent interaction with hydrochloric acid, measured using the calorimetric method. The working hypothesis for the laboratory is the assumption that the final thermal effect of the entire process will determine the exothermic reaction, which means that the energies of the resulting substances will be lower than the energies of the initial reagents.
Methods and Materials
This laboratory work was performed virtually on the ChemCollective platform, but all the same steps can also be performed under actual laboratory conditions. Fifty ml of distilled water was transferred to a graduated cylinder; the initial temperature was recorded in Table 1. Exactly 1.00 g of solid sodium hydroxide was weighed in a foam cup on a scale, after which all the water (50 mL) was added to the sample — the maximum temperature reached by mixing was recorded in Table 1. For the second reaction, all the same procedures were performed, but 50 mL of hydrochloric acid was used instead of 50 mL of distilled water. Finally, for the third reaction, 25 mL of liquid 1M sodium hydroxide and 1M hydrochloric acid were premixed: the initial and maximum final temperatures were recorded in the table.
Results
Table 1 contains the measured temperatures of the three reactions as well as the results of the indirect calculations. Based on the measured temperatures, three values of their differences, ∆T, were determined, and the number of moles of alkali used was calculated. The equation for the heat of reaction q was modified by adding a minus sign to make the answer equal to the heat effect ∆H, after which all known values were substituted, and the heat values were calculated. Finally, to calculate the reaction enthalpy change ΔHrxn, the heat values obtained were divided by the mole quantities of the substance, giving the enthalpy change per mole of alkali; all results of the calculations are shown in Table 1. Below are detailed demonstrations of the heat calculations for each of the three reactions:

Table 1. Results of direct measurements (columns 1, 2) and calculations (columns 3-6) for three reactions.
It is worth clarifying that reactions 1, 2, and 3 are dissociation processes, and neutralization of alkali, which can be represented in pure ionic form:
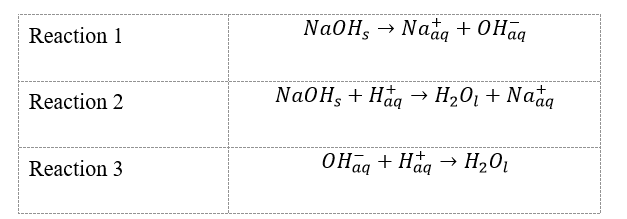
Summarizing the data in Table 1 and the pure ionic equations, one can conclude that to convert one mole of solid NaOH into the form of charged Na+ particles and to form water, it takes -102.0 kJ in the second reaction, which is the experimental value; for the third reaction, the enthalpy change is -57.6 kJ mol-1, which follows that this amount of energy is required to form one mole of water from the corresponding components. According to Hess’s law, these data can be used to obtain the net heat energy of the entire process:
From the calculations it follows that the assumed value of the heat of reaction is -102.8 kJ mol-1, and the percentage difference:
There is an extremely low error of 0.78% between the accepted and experimental heat values.
Discussion
This laboratory work aimed to determine the heat of the reaction of dissolution of solid alkali and further neutralization in hydrochloric acid using the calorimetric method. The results showed that the total heat of the reaction was equal to 0.8 kJ mol-1, from which it follows that the reaction was endothermic, which contradicts the working hypothesis of a negative value of the thermal effect of the reaction and, thus, of an exothermic process (LT, 2022).
It is well known that neutralization reactions are exothermic, which means that heat is released during them, and the heat effect of the reaction is negative (CCRI, n.d.). At the same time, salt dissolution reactions are mostly endothermic, requiring energy to dissociate the molecule into pieces, moving the ions further apart (Lu & Murray, 2022). In these reactions used in the present laboratory work, both dissolution and neutralization processes took place. The resulting positive heat of the reaction may indicate a more intense solid alkali dissolution reaction, which required little more heat than was released during neutralization.
The result obtained is interesting in the context of considering a broader framework. In particular, single-step processes are rarely involved in chemical production but often require several reactions to be involved in the sequence. Differences in the heats of such reactions due to Hess’s law make it possible to influence the processes in such a way as to raise or lower the proportion of certain substances throughout the synthesis process, especially in the case of reversible reactions, according to Le Chatelier’s principles. In addition, for fine synthesis procedures, the determination of the net heat of the reaction is necessary for the design of chemical equipment to improve the efficiency and purity of production.
Overall, looking at the low percentage difference and consistency with the scientific results, it is safe to say that this laboratory work was successful. Aspects that may have affected the reliability of the results and thus should be taken into account in the future include the thoroughness of the measurements, the possibility of repeated tests to minimize errors, and the extrapolation of procedures to other substances in order to verify the results.
References
CCRI. (n.d.). Enthalpy of neutralization [PDF document]. Web.
LT. (2022). Enthalpy of reaction. LibreTexts. Web.
Lu, J. X., & Murray, J. (2022). Biochemistry, dissolution, and solubility. NIH NCBI. Web.