Abstract
This experiment was conducted in the histopathology laboratory to illustrate routine staining by use of special stains. The special stains used were the Masson’s trichrome and Congo red. The Masson’s trichrome is commonly used to reveal the architecture of tissues and in the preparation of frozen sections. This is carried out systematically using the necessary specimen that entails mainly paraffin sections. Congo red detects amyloid in tissues of patients with amyloidosis. Masson’s trichrome is for carrying out experiments that can help in the determination of collagen in tissues such as skin and heart for analysis and likely diagnosis.
It’s also used to draw a distinction between smooth muscle and collagen in tumors. The Masson’s trichrome stain was used in the staining of normal uterus and the uterus fibroid. The former turned pinkish red while the latter turned red-blue. On the other hand, Congo red stained a section of the spleen tissue where its components turned colorless and light pink. Methods of carrying frozen sections were illustrated. This was followed by staining of the slide and examination using the microscope. There was the fine adjustment of the microscope to help give a clear image from which the specimen’s diagram was observed.
Introduction
Haematoxylin and Eosin (H&E) are in most cases used to stain most tissue sections that are produced in a haematology laboratory, at least initially (Brakhage, Jahn and Schmidmt, 1990, p. 37). Nonetheless, H&E alone may be limited in the number of applicable cases to enable a pathologist be able to reach at a given diagnosis. Therefore, the pathologist may be required to request for other levels from immunohistochemistry, the block or special stains.
The term ‘special stains’ refers to all other stains rather than H&E and are suitable for some specimens (Bass, Carr and Boulay, 2004, p. 14). However, the number of stains that can be requested is quite great. Special staining is a routine requirement for some tissues. A number of other stains are used in the staining of most liver tissues. Examples of such stains include reticulin stains and Masson’s trichrome (Gershwin, 2007, p. 130).
“The trichrome stain also highlights Mallory hyaline and megamitochondria in alcoholic liver disease” (Macsween, 2007, p. 130). This is required in revealing the architecture of the tissue and any other evidence for the formation of nodule observed in cirrhosis, a PERLS stain for deposits of iron, a Chromotrop-Anilinblue (CAB) stain, a Periodic Anti-Schiff (PAS) applicable in detecting the alpha1 – antitrypsin bodies observed in the genetic disorder alpha1- antitrypsin deficiency. Both Giemsa and H&E will be suitable for staining biopsies from the stomach (Odze and Goldblum, 2009, p. 123; Fudge, 2000, p. 233).
The former will reveal Helicobacter – a type of bacteria that is commonly causes stomach ulcers and gastritis (Gislason and Alpha Nutrition Health Education, 2003, p. 73; Turkington and Ashby, 2007, p. 141). The two commonly used special stains in this practical are the Congo red and the Masson’s trichrome. They will be used in staining some tissue sections and in revealing some important diagnostic features.
This method can be applicable to detect collagen fibres in some tissues for example heart and skin tissues on sections that are paraffin embedded and formalin fixed. At times, they may be used on frozen sections also (May and Schaitkin, 2000, p. 154). The method is also used to draw a distinction between smooth muscle and collagen in tumors and in collagen’s increase in sicknesses such as cirrhosis. “Trichrome stains are used mainly to differentiate collagen and muscle fibres and two of the most frequently used as far as skin is concerned are Van Gieson’s and Masson’s trichrome” (Marks, Dykes and Motley, 1993, p. 137).
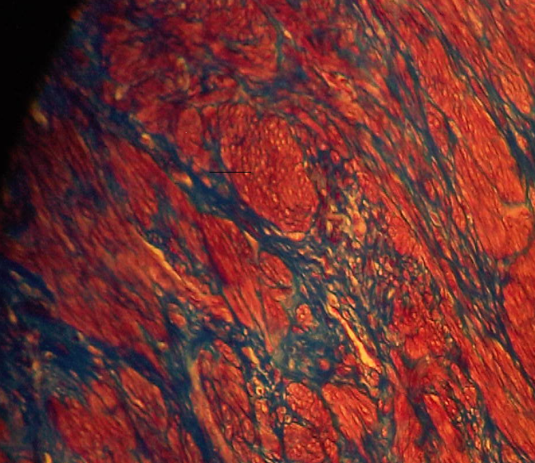
For kidney and liver biopsies, it acts as a routine stain. The nuclei will be stained black, collagen fibres blue, whereas red is the color that muscle, cytoplasm and erythrocytes will be stained to. On the other hand, Congo red is a stain that has a high PH. It is used to detect amyloid in paraffin embedded tissue of patients who have amyloidosis (Burnett and Crocker, 2005, p. 24). It can still be applicable in frozen sections. The nuclei will be stained blue while the amyloid will be stained red. Congo red can be used in the detection of eosinophils (Howie, 2001, p. 14).
Most of the staining on amyloid by the Congo red special stain seems to be dependent on the configuration of dye. The Congo red dye being a molecule that is linear has a configuration that allows hydrogen bonding of the amine and azo groups of this dye to amyloid’s hydroxyl radicals that are equally spaced. Some methods allow for the pre-treatment of the section with alkali that helps in enhancing internal hydrogen bonding between protein chains that are adjacent. Consequently, these methods provide for more potential sites present for the bonding of the dye. A counterstain especially hematoxylin is normally carried out and ends up coloring the nuclei. After staining with Congo red, amyloid gets berefringent (Anon, 2006, p. 2).
Methodology for use of Masson’s trichrome
The sections of the specimen were deparrafinized and rehydrated through 100%, 95% and 70% alcohol and then washed in distilled water.
The solution of Weigert’s iron hematoxylin was used to stain it for a period of 10 minutes.
Prior to washing it in distilled water, running tap water was used to rinse it for a period of ten minutes.
It was then stained in Biebrich scarlet acid, fuchsin solution for 15 minutes. This was followed by washing in water.
The specimen was differentiated in phosphomolybdic-phosphotungstic acid solution for 15minutes.
There was a transfer of the section to the aniline blue solutions and while there, it was stained for 10 a duration of minutes.
Distilled water was then used to rinse it after which it was differentiated into 1% acetic acid solution for a minute.
The specimen was then washed in distilled water and dehydrated quickly through 95% ethyl alcohol.
Resinous mounting medium was then used in the mounting of the specimen.
The slide was then examined under a microscope.
The image observed through the microscope was then drawn and well labeled.
Methodology for use of Congo red
The sections were deparrafinized and hydrated to distilled water. A working solution of Congo red was then used to stain them for 10 minutes.
After rinsing in distilled water, they were differentiated quickly in an alkaline solution.
They were then rinsed in tap water and counterstained with in Gill’s hematoxylin in 30 minutes.
Tap water was used to rinse the solutions before being dipped in a solution of ammonia until they had to change to blue.
They were rinsed in tap water for 5 minutes and dehydrated through 95% and 100% alcohol.
They were then cleared in xylene and mount with a mounting medium.
The slide was then observed under a light microscope.
Results
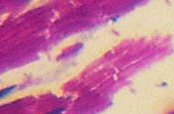
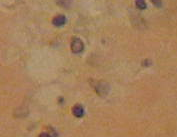
The results reveal that the uterus fibroid has cytoplasm, keratins and erythrocytes that appear red when stained using Masson’s trichrome. Collagen appears blue whereas the nuclei are black. The control uterus appeared to be pinkish red in its components with the cytoplasm appearing pink red whereas the nuclei had a blue – black colour. On the other hand, the spleen tissue had its nuclei appearing pale pink while its amyloid and elastic tissues appeared colorless. The slides of the experiment are shown below
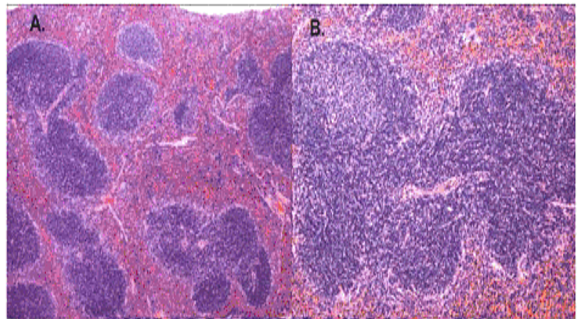
Normal histological structure of the spleen tissue
Discussion
“In a normal spleen section, the white pulp is supported by a network of dendritic and reticular cells organized in several distinct lymphoid zones: the T-lymphocyte –rich central zone, surrounding a central arteriole, adjacent mantle zone of small B lymphocytes and outer marginal zone of B lymphocytes” (Li, 2008, p. 50).
Masson’s trichrome stain is used to evaluate portal tract structures such as veins, bile ducts and arteries. Besides, it is a stain that is applied in the assessment of fibroids. Additionally, the stain highlights megamitochondria and Mallory hyaline in liver disease that’s caused by alcohol (Mahtab, 2009, p. 70).
Fibroids are leiomyomas that occur in the uterus. They are composed of smooth muscle fibres in interlacing bundles that are interconnected by fibrous connective tissue in varying amounts. The nuclei are spindle shaped with ends that are vascular and blunt. Due to fascicular arrangement in planes that vary, the fibre bundles seem to form whorls (Rajesndran, 2009, p. 188). Differentiated cells have myofibrils that are numerous, well oriented and appear as deep red. They appear to have parallel lines that are longitudinally placed and run the length of the cell when stained using Masson’s trichrome (Weiss, Goldblum and Enzinger, 2001, p. 729).
When a spleen tissue section is stained using Congo red, amyloid appears to be orange red when observed through normal light. However, when viewed under light that is polarized, it imparts an apple green birefringence to the material (Jones, Hunt and King, 1997, p. 50). However, the nuclei appear blue. This is a property that all β – pleated proteins tend to share and it seems to be particular to that given conformation (Bancroft and Gomble, 2008, p. 273).
“Amyloid is the deposition within tissues of a group of pathological proteins which form characteristic β pleated sheets” (McCarthy, Hacking and Al Mufti, 2004, p. 155). It comprises of a fibrillar ultrastructure and serum protein A and P are the proteins that constitute it. It is also comprised of immunoglobulin light chains and peptide hormones. It may either be local or systemic and it does interfere with the tissue or organ that has been affected, normally through the accumulation of the basement membrane.
Histologic staining using Congo red is what characterizes all forms of amyloid; hence this remains a requirement for diagnosing the disease (Gertz and Greipp, 2004, p. 158).
Other Stains used in the histopathological lab
Apart from the Congo red, other stains used in the staining of amyloid are H&E (Scully et al, 2010, p. 7) Melzer’s reagent, Indian ink and lactophenol (Bell, 1983, p. 51).
This bright apple – green birefringence that is achieved when Congo red stain is used is not only highly selective but also can be easily seen and has therefore been termed by many specialists as the most dependable characteristic of amyloid in the contemporary use (Bancroft and Gomble, 2008, p. 273). In addition, due to its visible outcome, when viewed under ultra violet light, Congo red staining can enable one to see very small amyloid deposits that other special stains may not achieve (Flores, 2010, p. 686).
On the other hand, Giemsa staining is applicable when staining blood rather than cultural cells. Its procedure is simple but it gives rise to polychromatic stain that is o0f high contrast. However, cells may have spotted appearance due to precipitated stain (Freshney, 2010, p. 249).
Elastic Van Gieson stain is applicable in the identification of vessels that are blocked. Through its use, a pathologist can easily make a distinction between past fibrosis and recent collapse. This is because only the former is positive. Making these differences may be difficult when using other stains such as reticulin and H&E (Scheuer and Lefkowitch, 2006, p. 15).
Studies have revealed that PAS stain is a tool that can be used to detect glycoproteins within sections of tissues. Its carbon- carbon bonds cleave by use of a periodic acid to form aldehyde groups. These can then form a red complex by reacting with leucofuchsin. In histochemistry, the stain has been found to well only for compounds that are of high molecular weight. This means that it may not detect low molecular weight compounds (National research Council, 1966, p. 84).
“PAS stain has also been reported as a satisfactory stain for fungi in plant tissue” (Muller, Bills and Foster, 2004, p. 254). This stain also works well for dermatophytes (Braun-Falco, 2000, p. 316).
Moreover, PERLS stain is used to check for the availability of ferric iron in some sections of the brain. It works together with hydrochloric acid to give rise to a precipitate that is blue in color that is commonly called Berlin blue (Yehuda and Mostofsky, 2009, p. 224).
Conclusion
Having successfully performed the experiment to achieve the desired result, the practical thus met its objectives. The application of Congo red and Masson’s trichrome stain was well appreciated.
Reference List
Anon. 2006. Congo red Stain. Web.
Bancroft, J. and Gomble, M. 2008. Theory and Practice of Histological Techniques. Philadelphia: Elsevier Health Sciences. Web.
Bass, P. Carr, N. and Boulay, C. 2004. Pathology: a core text of basic pathological processes with self-assessment. NY: Elsevier Health services. Web.
Bell, A. 1983. Dung fungi: an illustrated guide to coprophilous fungi in New Zealand. Wellington: Victoria University Press. Web.
Brakhage, A. Jahn, B. and Schmidmt, A. 1990. Aspergillus fumigatus: biology, clinical aspects, and molecular approaches to pathogenicity. Basel: Karger Publishers. Web.
Braun-Falco, O. 2000. Dermatology. NY: Springer. Web.
Burnett, D. and Crocker, J. 2005. The Science of Laboratory Diagnosis. West Sussex: John Wiley and Sons. Web.
Flores, A. 2010. Comparative study of Congo red fluorescence and immunohistochemistry in cutaneous amyloidosis. Romania Journal of Morphology and Embryology, 2010, 51 (4). Web.
Fudge, A.M. (2000). Laboratory medicine: avian and exotic pets. Philadelphia: Elsevier Health Sciences. Web.
Freshney, R. 2010. Culture of animal cells. NJ: John Wiley and Sons. Web.
Gershwin, E. 2007. Liver Immunology: Principles and Practice. NJ: Humana Press. Web.
Gertz, M. and Greipp, P. 2004. Hematologic malignancies: multiple myeloma and related plasma cell disorders. NY: Springer. Web.
Gislason, S. and Alpha Nutrition Health Education, 2003. Food and Digestive Disorders. Sechelt B.C, Canada: Environmed Research. Web.
Howie, A. 2001. Handbook of Renal biopsy Pathology. Dordrecht: Kluwer Academic Publishers. Web.
Jones, T. Hunt, R. and King, N. 1997. Veterinary Pathology. Baltimore: Williams and Wilkins. Web.
Li, S. 2008. Mouse models of human blood cancers: basic research and pre-clinical applications. NY: Springer. Web.
MacSween, R.N. 2007. MacSween’s Pathology of the Liver. NY: Elsevier Health services. Web.
Mahtab. 2009. Liver: A complete book on Hapato- Pancreato- Biliary Diseases. Chennai: Elsevier. Web.
Marks, R. Dykes, P. and Motley, R. 1993. Clinical signs and Procedures in Dermatology. London: Taylor and Francis. Web.
May, M. and Schaitkin, B. 2000. The Facial Nerve. NY: Thieme. Web.
McCarthy, K. Hacking, M. and Al Mufti, R. 2004. Vivas and communication skills in surgery. Philadelphia: Elsevier Health Sciences. Web.
Muller, G. Bills, G and Foster, M. Biodiversity of fungi: inventory and monitoring methods. NY: Elsevier. Web.
National Research Council (US). 1966. Wound healing: Proceedings of a workshop conducted by the Committee on Trauma. National academies. Web.
Odze, R. and Goldblum, J. 2009. Surgical pathology of the GI tract, liver, biliary tract, and pancreas. Philadelphia: Elsevier Health Sciences. Web.
Rajesndran, 2009. Shafer’s Textbook of Oral Pathology. Noida: Elsevier India. Web.
Scheuer, P. and Lefkowitch, J. 2006. Liver Biopsy Interpretation. Philadelphia: Elsevier Health Sciences. Web.
Scully et al. 2010. Oral Medicine and Pathology at a Glance. West Sussex: John Wiley and Sons. Web.
Turkington, C. and Ashby, B, 2007. The encyclopedia of infectious diseases. NY: InfoBase Publishing. Web.
Weiss, S. Goldblum, J. and Enzinger, F. 2001. Enzinger and Weiss’s soft tissue tumors. NY: Elsevier health Sciences. Web.
Yehuda, S. and Mostofsky, D. 2009. Iron Deficiency and Overload: From Basic Biology to Clinical Medicine. NY: Springer. Web.