Introduction
The scientific community has always speculated about the origin of life on earth. Theories such as the formation of life through the fusion of chemicals in hydrothermal vents on the ocean floor or in caverns deep below the planet’s crust have dominated scientific discussions. The idea that inorganic molecules could precipitate organic substances under the right conditions prompted the development of experiments designed to explore the concept. The basis of the Miller-Urey experiment was to establish the fact that inorganic matter was capable of producing amino acids, which are essential for life. The biologists theorized that the earth’s early atmosphere, which was mostly composed of hydrogen, water, methane, and ammonia had the capacity to form the building blocks of organic matter. The idea is supported by research findings that demonstrated that carbon dioxide, nitrogen, water, and oxygen were not part of the earth’s early atmosphere (Miller, 1953). The hypothesis was tested by the construction of an apparatus designed to circulate the components of the earth’s early atmosphere through an electric discharge.
The Experiment
The scientists sealed compounds that mimicked earth’s early atmosphere in sterile glass tubes and flasks that were linked in a loop and circulated within the experiment’s apparatus, as illustrated in Figure 1 below. The objective was to create conditions that represented the atmospheric composition of the earth soon after it had formed. One flask was filled to the halfway point with water, while the other was mounted with a pair of electrodes. The water was then heated to produce vapor, which was then added to the chemical mixture. The formed gases were then circulated around the apparatus in a move designed to mimic the earth’s early atmosphere. The U-tube was designed to limit circulation in the opposite direction and avoid potential contamination.
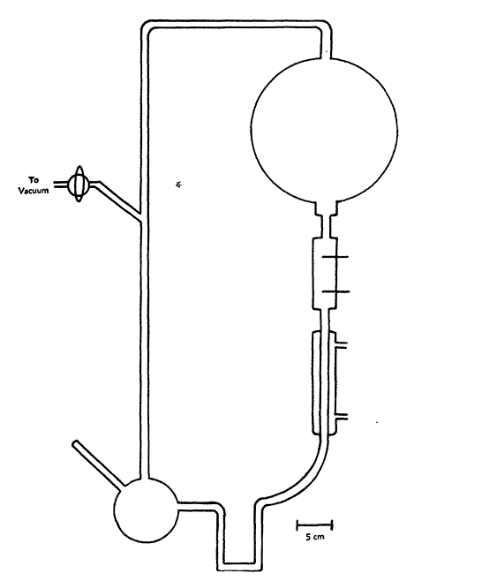
The experiment involved specific steps designed to facilitate the achievement of the desired results. The procedure, as documented by Miller (1953) involved the sealing of the opening of the boiling flask after the addition of 200mls of water, eliminating the air, adding 10cm pressure of hydrogen, 20 cm pressure of methane, and 20 cm pressure of ammonia (Miller, 1953). The water was then boiled, after which the gas mixture was allowed to circulate for seven days (Miller, 1953). The researchers noted that the water in the flask developed a pink hue after the first day and became significantly red and turbid after seven days (Miller, 1953). It is worth noting that the turbidity was a result of the colloidal silica found in the glass, while the red color was the result of the silica’s absorption of organic compounds (Miller, 1953). The researchers noted the presence of yellow organic compounds in the analyzed solution.
Experiment Results
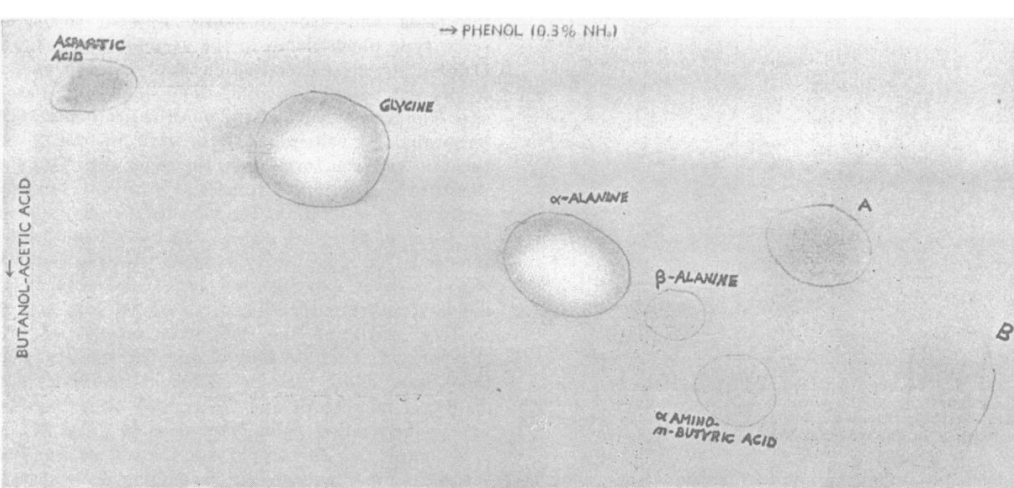
The assessment of the solution from the flask led to the identification of specific amino acids. The scientists noted that glycine, β-Alanine, and α-Alanine were identified from the sample (Miller, 1953). Weak spots on the paper chromatogram were noted to be aspartic acid and α-amino-n-butyric acid (Miller, 1953). The researchers note that their experiment was designed to mimic the primitive earth’s atmosphere and not the ideal conditions required for the development of amino acids. The researcher noted that while the yield from the experiment was little compared to the total energy expenditure, it is likely that a more efficient apparatus could produce amino acids on an industrial scale. The results are demonstrated in Figure 2, which is a paper chromatogram.
Discussion
The water in the flask simulated the planet’s surface water, and the vapor served as the evaporation process from large water bodies. The electrodes acted as the lightning that frequently struck the earth’s surface as it formed. The electrical discharge was used to generate free radicals rather than ultraviolet radiation in view of the fact that quartz was capable of absorbing wavelengths that were capable of initiating photo-dissociation in the gas mixture (Miller, 1953). The scientists cooled the vapor, and the resultant condensation was analyzed.
The scientists noted that the amino acids created in the discharge were not volatile, which resulted in their accumulation in the water phase. They also noted that the slow circulation of the gas mixture was an advantage since they noted reduced production in an alternate apparatus fitted with an aspirator designed to boost circulation (Miller, 1953). At the experiment’s conclusion, the solution in the flask was removed and treated to prevent the growth of organisms. The researchers used paper chromatography to determine that the amino acids detected in the solution were the direct result of the interaction of the elements in the apparatus. Possible contamination by living organisms was discounted by the fact that the water was boiled and the solution treated to prevent organic growth.
Conclusion
The experiment demonstrates that it is indeed possible to generate organic molecules from inorganic components that are thought to have constituted the earth’s primitive atmosphere. This scientific exploration of the origin of life formed the foundation upon which numerous other studies exploring the same idea were based. While there is some debate regarding the exact composition of earth’s early atmosphere, the experiment served as a gateway into the exploration of numerous other conditions under which life could have formed. The Miller-Urey experiment proved that life’s complex molecules could have arisen through simple chemical reactions made possible by a combination of factors on the newly formed planet.
Reference
Miller, S. L. (1953). A production of amino acids under possible primitive earth conditions. Science, 117(3046), 528–529. Web.