Introduction
The following paper is going to discuss the prototypes of different drugs belonging to different pharmacological and chemical classes. The prototypes of the chemical classes of Cinnarizine C26H28N2, Diltiazem C22H26N2O4S, Nitroglycerin C3H5N3O9, Hydralazine Hydrochloride C8H8N4, Losartan C22H23ClN6O, and Tacrine C13H14N2, are going to be evaluated from different aspects like structural analysis, acid-base characteristics, ionization processes, polarity, solubility, partition and possible theoretical metabolic pathways. The following pages are going to elaborate on the biopharmaceutical features of these drugs at molecular level.
Drug Classification
Cinnarizine. Originally it is classified as an anti-histaminic which is used for controlling vomiting caused by motion sickness. He principle of its action is closely associated with the signal transmission between vestibular apparatus of the inner ear and the vomiting centre of the hypothalamus.
Diltiazem. As O’Connor (2004, p. 349) emphasizes, “it is a non-DHP member of the benzothiazepines group. These are the calcium channel blockers, which are generally used for treatment of hypertension, angina pectoris, and some types of arrhythmia”. It is also regarded as a preventive medication for migraine.
Nitroglycerin is used to treat heart deceases such as angina and chronic heart failure.
Hydralazine Hydrochloride. In accordance with Reines (200, p. 712): relaxes vascular smooth muscles of arteries and arterioles, causing peripheral vasodilation and decreasing peripheral vascular resistance. These actions decrease blood pressure and increase heart rate, stroke volume, and cardiac output.
Losartan. This is used for treating the problems of high blood pressure
Tacrine is a parasympathomimetic and a centrally acting cholinesterase inhibitor (anticholinesterase). It was the first centrally-acting cholinesterase inhibitor approved for the treatment of Alzheimer’s disease. (Reines, 2000)
Metabolism and Absorption
Molecular Properties
Cinnarizine
Mol. mass 368.514 g/mol
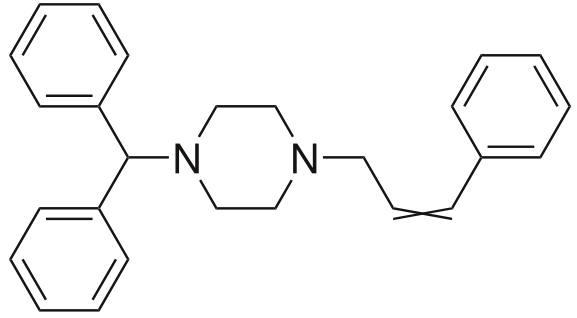
As for the matters of covalent bonding, and the issues of alkylation, it should be stated that in water-methanol solutions (18.7 – 56.1% of methanol), making use of aqueous pKa values interpolated from pKa extrapolations. During such experiments, cinnarizine behaved as a non-chaser. Aqueous solubility of 0.34μM was determined by extrapolation. (Foye, Lemke, Williams, 2007)
A covalent bond:
- (C-|-C8)-alkylene-,
- (C2-C-I o)-alkenylene-, or 4) -(C3-C8)-cycloalkylene-,
- C-i-CβJ-alkyl.
These are close to β-Dicarbonyl groups. As previously stated, the “X” atom of a β-dicarbonyl group can be either carbon or nitrogen. The β-Dicarbonyl may exist in either the keto or enol tautomeric form. Resonance stability dictates which of these two forms is predominant. In the case of warfarin, tetracycline and many other drugs, it is the enol form which is predominant and thus the compound is drawn in that manner. While you are not expected to predict which form would predominate for a given compound, you will need to be able to identify enol forms of β-Dicarbonyl. (Reines, 2000)
As Foye, Lemke and Williams (2007, p. 395) emphasize in the research:
A protective effect occurred at low concentrations (<50 μM) of Cinnarizine and was accompanied by the inhibition of NAD(P)H oxidation and the restoration of the mitochondrial membrane potential decreased by a high concentration of Ca2+ (25 μM). However, at higher concentrations (>50 μM) of cinnarizine and flunarizine and in the absence of both tert-butylhydroperoxide and Ca2+, their effects on the mitochondria were reversed as follows: mitochondrial permeability transition was generated, mitochondrial NAD(P)H was oxidized and membrane potential collapsed. These deleterious effects were not antagonized by cyclosporine A, the most potent inhibitor of the mitochondrial permeability transition, but by 2,6-di-tert-butyl-4-methylphenol, a known antioxidant agent.
Diltiazem
Mol. mass 414.519 g/mol
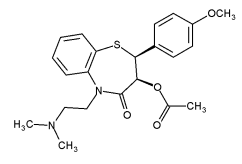
Similar to what was discussed with the β-dicarbonyl compounds, the functional group must contain an ionizable proton. In this case, the nitrogen atom of the sulfonamide functional group has been acetylated (highlighted in red). Thus, it no longer contains an acidic proton and therefore is not acidic. It is still a sulfonamide (i.e., it is a “sulfur-containing” amide), but since the nitrogen contains a substitution, the functional group is neutral. (World Health Organization, 2001)
The optically active isomers of the molecule are featured with useful pharmacological and therapeutic characteristics as anti-emetics and anti-cataleptics in some ways are quite different from those of the racemic mixture. Originally the optical rates are represented in the following table:
As for the issues of intermolecular drug binding interactions, it should be stated that in accordance with Adjepon-Yamah (2003, p. 145) “the standard formation (p = 0.1 MPa) of crystalline thioxanthene (117.4 ± 4.1 kJ·mol−1) was defined only by the means of the investigational standard molar energy of combustion in oxygen. Thus, the experiment presupposed the measuring by rotating-bomb calorimetry at T = 298.15 K”
A covalent bond, 2) -(C2-C10) alkenyl,
- (C2-C10) alkynyl,
- (C0-C4) alkylene CH(OH)-(C0-C4) alkylene,
- (Co-C4) alkylene 0 (Co-C4) alkylene,
Nitroglycerin
Mol. mass 227.09 g/mol−1
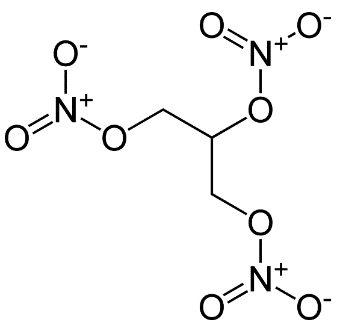
The fact is that, the relation to the hydroxyl group makes the Nitroglycerin molecule polar. Thus, this group is able to form hydrogen bonds to one another and to other compounds. In the light of this fact, it should be emphasized that such bonding of nitrogen means that Nitroglycerin can be used as protic solvents. (O’Connor, 2004) As Reines (2000, p 416) claims, “two opposing solubility trends in alcohols are: the tendency of the polar one to promote solubility and of the carbon chain to resist it.”
The rates of the optically active isomers of the molecule are represented in the following table:
Hydralazine Hydrochloride
Mol. Mass 236.14 g/mol
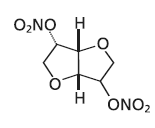
Originally, Hydralazine Hydrochloride is soluble in organic solvents such as alcohol, chloroform and ether. It cannot share the negative charge with an adjacent carbonyl, sulfonyl, or aromatic ring, thus, it is not fully soluble in water. As a result the proton is not ionizable and the functional group is not acidic.
The optically active isomers of the molecule possess useful pharmacological and therapeutic properties as anti-emetics and anti-cataleptics in some ways quite different from those of the corresponding racemic mixture. Originally the rates are represented in the following table:
Losartan
Mol. mass 422.91
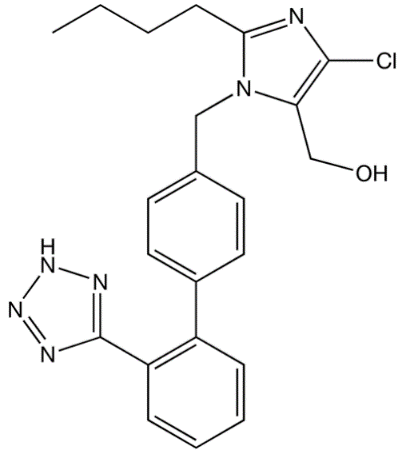
First of all it should be sated that the rings of Tetrazole chemical structure are isosteres of carboxylic acids. Thus, as Foye, Lemke and Williams (2007, p. 218) emphasize: “The lone hydrogen in this ring system is acidic. the resulting negative charge can be equally shared among all five atoms of the tetrazole ring. In comparison with a carboxylic acid, a tetrazole ring is less acidic (pKa value 6) and more lipophilic.”
The optically active isomers are quite different from those of the corresponding racemic mixture. Originally the rates are represented in the following table:
Tacrine
Mol. mass 198.264 g/mol
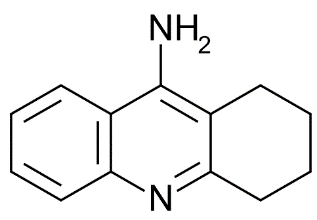
Imines are unsaturated amines. One example is in the benzodiazepine class of compounds. As a rule, imines are less basic than their corresponding amines. This term refers to the presence of double or triple bonds. Unlike single bonds, these are not “saturated” with hydrogen. The nitrogen atom of the sulfonamide functional group has been acetylated (highlighted in red). Thus, it no longer contains an acidic proton and therefore is not acidic. It is still a sulfonamide (i.e., it is a “sulfur-containing” amide), but since the nitrogen contains a substitution, the functional group is neutral.
The optically active rates are represented in the following table:
References
Adjepon-Yamah, K.K., Scott, D.B. & Prescott, L.F. (2003). “Impaired absorption and metabolism of oral lignocaine in patients undergoing laparoscopy” Br. J. Anesth., 45, 143-147.
Foye, W. O., Lemke, T.L., Williams, D.A. (2007) “Foye’s principles of medicinal chemistry” Lippincott Williams & Wilkins
O’Connor SE, Grosset A, Janiak P. (2004) The pharmacological basis and pathophysiological significance of the heart rate-lowering property of diltiazem. Fundamental and Clinical Pharmacology. 14 (2):145-53.
Reines, Brandon P (2000). “The Relationship between Laboratory and Clinical Studies in Psychopharmacologic Discovery”. Perspectives on Medical Research (Medical Research Modernization Society) 2.
World Health Organization. (2001) “Guidelines for ATC (Anatomical Therapeutic Classification)” Oslo: World Health Organisation Collaborating Centre for Drug Statistics Methodology,.