Introduction
Explanation of the mechanism of natural phenomena from the point of view of scientific concepts is of great importance for the academic community, as it helps to establish the essence of the world systems organization. Thus, even behind the most unobvious appearances, such as the color of the sky at different times of the day.
There are complex physical and chemical processes. An in-depth study of these mechanisms largely determines the extent of understanding natural changes and allows manipulation of this knowledge to predict specific results. Thus, the phenomenon of sky coloring at different times of the day is traditionally associated with the physical phenomenon of scattering the light beam emitted by the Sun. This paper will discuss in detail the mechanics of this process and demonstrate why the change in time of day, which is equal to a change in the planet’s position around a star, affects the color of the sky. This work aims to summarize the available data on the physical component of light scattering in the context of determining the causes of blue and reddish-orange sky color.
Background Information
The uniqueness of the compositional structure of the planet Earth is determined by the sufficient distance from the system-forming star, the Sun, which determines the possibility of the existence of the atmospheric layer. In other words, if the Earth were closer to the Sun, as is typical, for instance, of Mercury or Venus, the atmosphere of the celestial body would be eliminated due to the strong radiation wind emitted by the star and high temperatures. Thus, planet Earth has a gas shell rich in chemical elements: the basis of the atmosphere is molecular nitrogen, N2, (78%), oxygen, O2, (21%), argon, Ar, (0.93%), carbon dioxide, CO2, (0.04%), and other gases (<0.03%) (Sharp, 2017). In combination with a huge number of water molecules, atmospheric chemicals form a grandiose set of compounds inhabiting the Earth’s gas layer in a heterogeneous manner. It is these molecules that, together with the environment, form a suspension composition of variable density, which becomes the main obstacle to the sunlight.
Of great importance in the discussion of this physical phenomenon is the recognition of the fact that light has a dual structure. Although this has been a debate in the scientific community for centuries, modern science clearly recognizes that light is both a particle and a wave, which defines the dualistic properties of electromagnetic radiation (Rashkovskiy, 2016). As a wave, light has its own wavelength, can create phenomena of diffraction and interference when interacting with obstacles. At the same time, as a particle, light is a flow of photons that propagates in a straight line from source to object. Taken together, both properties produce a combination of particle and wave effects, making the light beam a unique phenomenon that can also be dispersion.
It is necessary to discuss further what the nature of color is and how light is associated with a variety of observed tones. The observed white light itself is a mixture of all available color variations, including red, green, and blue, on which all other shades are integrated. White light can be subject to physical dispersion, resulting in a decomposition of the spectrum, as illustrated in Figure 1. It is known that the difference between colors is the length of the corresponding waves (“Visible spectrum,” 2019). Thus, a good example that justifies this effect is the existence of a rainbow: when sunlight hits water droplets, the rays refract and break down into different color shades.
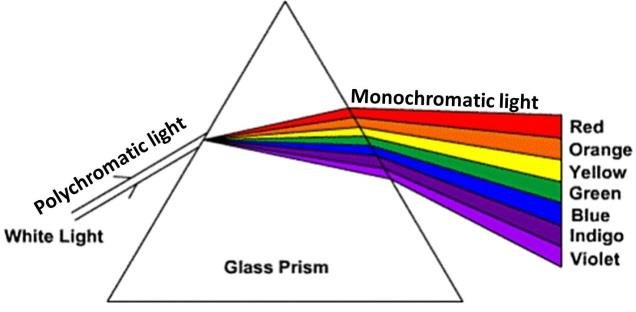
Moreover, every object in the Universe actually has no color shade of its own but has a molecular or atomic structure. When the rays of polychromatic light illuminate an object, most of them are absorbed by molecules, but only some wavelengths are reflected from the particles due to their comparable size. The reflected light is thus what the human eye sees: the color of an object.
Reasons for the Blue Sky
The blue color of the sky, which is mainly observed during the day time, is dictated by the phenomena of light waves scattering on solid particles of the atmosphere. The electromagnetic beam of white light coming from the Sun is directed towards the Earth’s surface, but on this way, there is an obstacle in the form of a gas envelope of the celestial body. As it was mentioned earlier, some wavelengths of the color spectrum pass freely through this obstruction, while some of the beams meet proportional atmospheric molecules. For instance, frozen nanoscopic crystals of water may have a similar size to that of the blue wavelength (about 4•10-4 nm). As a result of the coincidence of sizes, the blue color may be reflected from solids and fall into the human eye, which causes the blue color of the sky, as shown in Figure 3.
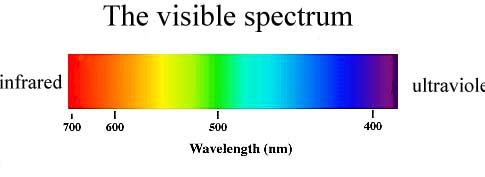
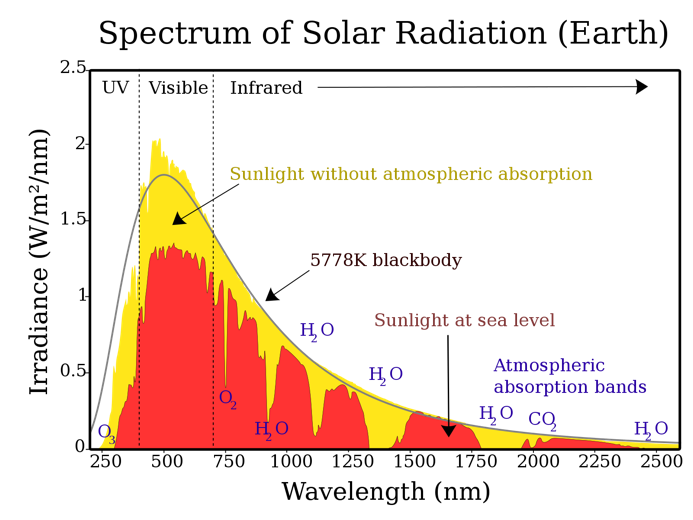
The justification for the scattering effect of the short-wave parts of the spectrum is due to the physical effect of Rayleigh scattering. This concept states that the intensity of diffused light is calculated more for shorter wavelengths, namely the violet and blue parts of the spectrum (Equation 1). However, the reference to Figure 2 gives a clear indication that the purple part of visible light has the lowest wavelengths, but — for some reason — the sky looks blue. The explanation for the apparent paradox is that the intensity of the Sun’s electromagnetic radiation is lower in the purple than in the blue, as shown in Figure 4. Second, the human eye is known to perceive blue more easily than violet, which is justified by the presence of blue photoreceptors in the retina (Kolb et al., 2020). Thus, two effects simultaneously increase the likelihood that blue rays will enter the human eye more easily, as seen in the sky. Hence, the blue sky during the daytime is explained by the scattering of light on the inhomogeneities of the air environment caused by fluctuations in the density of the medium and the presence of foreign particles.
Reasons for the Red Sky
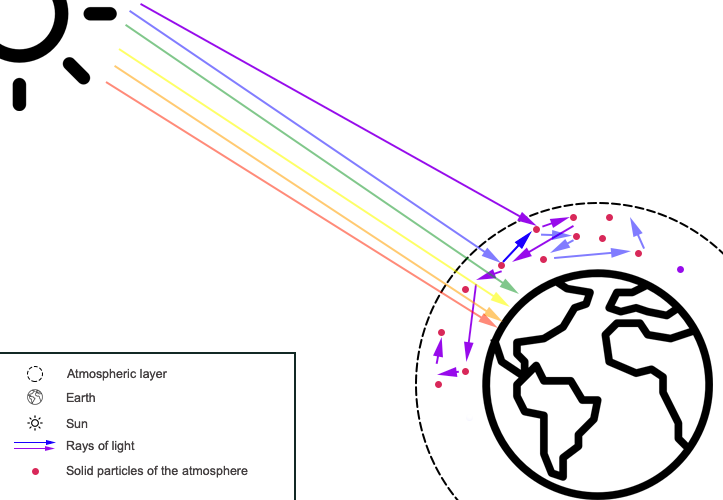
Therefore, the blue color of the sky indeed directly depends on the degree of homogeneity of the atmosphere and the nature of the molecules inhabiting the Earth’s gas envelope. This statement would not be in doubt if there were no dawn and dusk: the phenomena during which the sky is traditionally painted with reddish and orange colors. The explanation of this seeming violation of physical logic lies in the relationship between the distance between the source of radiation and the object. Thus, during the day, the Sun occupies different positions relative to the point of the Earth where the intended observer is. Consequently, the thickness of the atmosphere, which has to overcome the Sun’s rays, is also different, as shown in Figure 5. Thus, at noon, the rays follow a short path and fall at right angles.
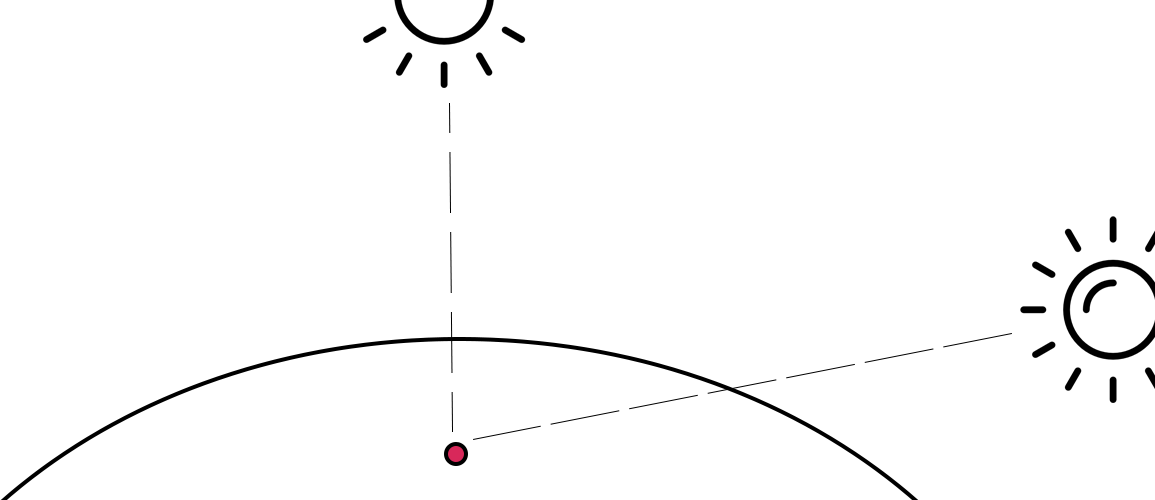
At the same time, being on the edges of the observer, the Sun emits rays that spread in the air environment tangentially, overcoming a much larger distance. In other words, on the way of sunlight, the beam encounters a large number of obstacles, which means that a huge amount of rays are scattered along the way. This effect justifies the fact that most of the blue and green spectrum is reflected to the sides, and the direct light beam containing the outstanding shades colors the sky in reddish tones. Furthermore, a large amount of particulate matter that inhabits the Earth’s atmosphere, and especially over cities with large amounts of emitted dust, intensifies the red glow of the sky. With a denser particle content, even the yellow and orange spectrum is difficult to reach the human eye.
Alternative Scenarios
The central determinants of sky color, as shown above, are the density and homogeneity of the gas envelope, its chemical composition, and the distance that the rays need to overcome on the way to the observer. The physical processes of dispersion and absorption of specific wavelengths are responsible for the apparent color effect of the air environment. Given the above, it would be fair to note that a change in any of the variables would have a significant effect on the shade of the sky. In particular, if the elementary composition of the gas envelope of the celestial body is different from that of the Earth, then one can assume that the sky there has a different color scheme.
The confirmation of the above hypothesis can be found, for example, in studies on the surface of Mars. It was shown that the sky of Mars in the daytime has a reddish-pink color, but during sunset and dawn, it is painted in blue (Perez-Hoyos, 2016). Probably, this effect is largely due to the composition of the gas envelope of Mars: it is dominated by dust. Thus, it is easy to see that this situation is the exact opposite of what can be observed on Earth.
Conclusion
Summarizing the above, it is worth repeating that the specific color of the sky is not an absolute parameter, but indeed, this illustrates those wavelengths that are reflected by solid particles of the atmosphere. Understanding the mechanism of the sky coloring in the shade should be preceded the determination of the chemical molecular composition of the atmosphere, the study of its density and homogeneity, and the measurement of the position around a star. It is important to remember that white light, with its wave-particle duality properties, is a combination of all existing sides of the visible spectrum, namely from purple to red.
Thus, the blue-violet zone has smaller wavelengths, while the orange-red zone is longer wavelengths. Due to the Rayleigh scattering effect, the white light beam passing through the planet’s gas envelope is scattered by collision with a large number of nanoscopic obstacles. This physical law states that short wavelengths will disperse more intensely than short wavelengths on average, and therefore the reddish rays are absorbed by the Earth’s surface, while the blue ones are reflected. The reflected light is what an observer sees when they look at an object. Meanwhile, the violet color is not observed in the atmosphere because, in the spectrum of solar electromagnetic radiation, this part is less intense than blue, as it was shown.
References
Chimot, J. (2018). Solar light interaction with the atmosphere. Homepage Julien Chimot. Web.
Kolb, H., Nelson, R. F., Ahnelt, P. K., Ortuño-Lizarán, I., & Cuenca, N. (2020). The architecture of the human fovea. Web.
Pérez-Hoyos, S. (2016). What color is the sky on Mars?Serious Science. Web.
Rashkovskiy, S. A. (2016). Quantum mechanics without quanta: The nature of the wave-particle duality of light.Quantum Studies: Mathematics and Foundations, 3(2), 147-160. Web.
Sharp, T. (2017). Earth’s atmosphere: Composition, climate & weather. SPACE. Web.
Syaodih, E., Suhandi, A., Maftuh, B., Hermita, N., Fratiwi, N. J., & Samsudin, A. (2019).Development and implementation of creative, solutive and smart teaching (CS2T) to improve 21st century capability on wave and optics[PDF document]. Web.
Visible spectrum. (2019). Michigan State University. Web.