Introduction
Sun has always been regarded as the key energy source for humanity since ancient times. The colossal energy and material reserve are able to supply light and warmth for millions of years, and the aim of humanity is to study how to use this energy wisely. Silicon cells that are also called solar cells are applied in various spheres of human life. The photovoltaic effect is aimed at transforming sunlight into electricity.
Various silicon compounds are used for creating solar panels; these are monocrystalline silicon, polycrystalline silicon, amorphous silicon, cadmium telluride, and copper indium selenide/sulfide (Lynn, 2010). The future of solar energy is regarded as the opportunity to deliver sunlight-generated electricity to every house and every industrial object, while contemporary technology presupposes low-efficiency output, and, regardless of the quick technological development, the dream of cheap and available electricity stays nothing more than a dream.
Solar Energy Usage
As it is stated in Goetzberger and Hoffmann (2005), the photovoltaic effect was first discovered by French physicist Becquerel. He noted that sunlight carries energy that may be converted into electricity. However, the first solar cell was invented in 1883 by Charles Fritts. He used the junction of gold and selenium, however, the efficiency of the construction was only 1%. Silicon was first used by Russian physicist Stoletov in 1905. As the effectiveness was essentially larger in comparison with Fritts’s experiment, silicon became the key component for photovoltaic cells.
Actually, this is explained by the statement that silicon has the optimal atomic structure, and is sensitive to sun radiation. However, certain alternatives are also used for increasing the effectiveness of the constructions and junctions. Hence, the actual importance of these alternatives is explained by using various components for improving silicon and non-silicon constructions. The key principle of the process is explained in Pachauri and Abeyratne (2000, p. 521):
“Electrons are ejected from a material’s surface upon exposure to radiation of sufficient energy. The photovoltaic effect is different in that the generated electrons are transferred between different bands (i.e. from the valence to conduction bands) within the material, resulting in the buildup of a voltage between two electrodes.”
Hence, the requirements to the atomic structure presuppose the existence of free electrons in atoms and the abilities for semiconducting. Hence, if sunlight affects the surface, the free-electron should be released from the atom, and transmitted to a conductor (electrode), for the electron did not have an opportunity to come back, the semiconductor is used, where the p-n junction is used.
Technical Process
The key difference factor of the solar cells is the material and technology that is used. Hence, the output parameters change, as well as the efficiency coefficient. First, it should be stated that the energy is transformed from the visible sunlight, and the technical features of the process are explained in Lynn (2010, p. 310):
“Many currently available solar cells are made from bulk material that is cut into wafers between 180 to 240 micrometers thick that are then processed like other semiconductors. Other materials are made as thin-films layers, organic dyes, and organic polymers that are deposited on supporting substrates. A third group is made from nanocrystals and used as quantum dots (electron-confined nanoparticles).”
In the light of this statement, it should be emphasized that the working regimes of solar cells are defined by the positioning of the cells towards the light, as the 90 angle presupposes the maximum effectiveness, while the lower angles decrease the effectiveness proportionally. Photons that are caught by solar cells undergo several steps.
There is no necessity to explain multi-step p-n junctions, as contemporary cells are created using a single p-n layer. Photons, carrying some particular energy charge, liberate the free electron from an atom. Schematically it is shown in Figure 1, where cell 1 is the photosensitive element, cell 2 is a semiconductor, and cell 3 is the electrode layer.
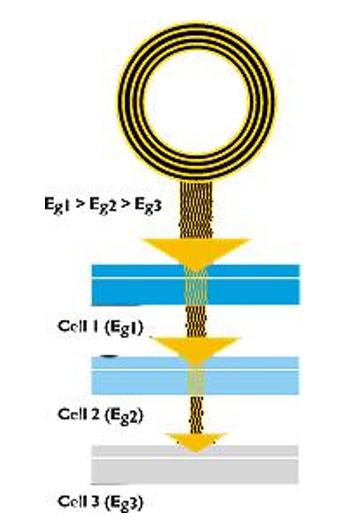
Photon behavior in a solar cell is defined by the materials used and the construction of the cell itself. Hence, there are at least three ways of photon behavior:
- Photon can pass directly to the silicon layer. In accordance with Lynn’s (2010) research, this happens if photons are not charged highly.
- Photon can reflect from the surface of the solar cell
- The photon may be absorbed. If this happens, the solar cell generates an electron-hole pair if the charge level of the photon absorbed is higher in comparison with the charge of the silicon bandgap value.
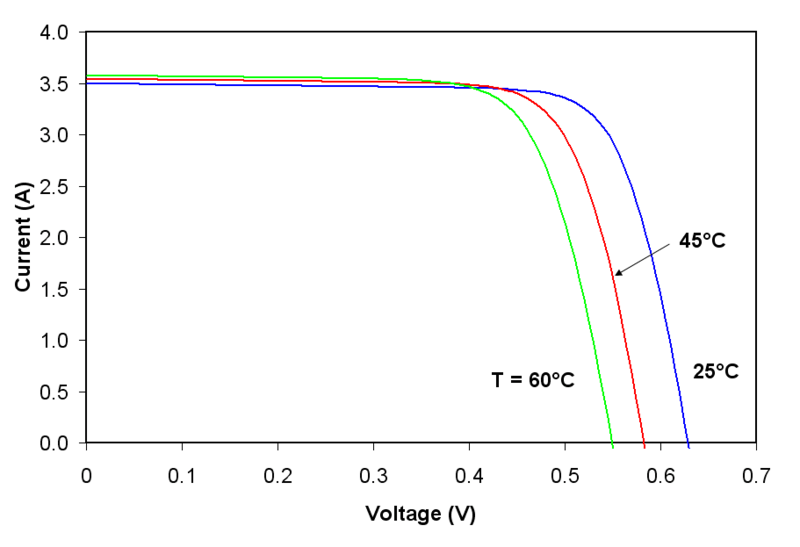
An additional factor for defining regimes is the temperature of the environment. The comparative values are given in Figure 2. However, the temperature defines the maximal output that may be derived from a single cell. It is in non-linear proportion with temperature and voltage.
An essential barrier for effective technology development is known as a physical side effect. It is explained by the statement given by Pachauri and Abeyratne (2000, p. 294):
“As cell characteristics are referenced to a common cross-sectional area, they may be compared for cells of different physical dimensions. While this is of limited benefit in a manufacturing setting, where all cells tend to be the same size, it is useful in research and in comparing cells between manufacturers. Moreover, the density equation naturally scales the parameter values to similar orders of magnitude.”
Considering the opportunity of current leakage, or loss depending on the temperature, another aspect of solar cell theory should be emphasized. This is called reverse saturation current, and it represents the reduction of Voltage with the increase of current produced. This is closely linked with the temperature behavior of solar cells, and the described saturation is regarded as the leakage caused by the transmission of currents across the p-n junction in reverse bias (Goetzberger and Hoffmann, 2005)
Ideality factor is the key parameter for defining the real capacity of a cell in comparison with the theoretic calculations. This is generally shown by voltage-current characteristics of the cell, and if the real characteristics coincide with the theoretic values, the ideality factor is 1. It may be higher or lower depending on space-charge region recombination.
In fact, most solar cells that are large enough in comparison with diodes, exhibit close to ideal behavior in Standard Test Condition (Pachauri and Abeyratne, 2000). However, depending on the space-charge region recombination, the device operation may be changed. The increase of the I parameter and the ideality factor may be regarded as the result of voltage increase, while the lower ideality factors may stimulate solar cell erosion.
Types of Solar Cells
The types generally depend on the materials used for manufacturing solar cells. Silicon is used as the basis for any photovoltaic material, while the types may differ. Hence, monocrystalline silicon is the most expensive, and effective enough. Multicrystalline silicon is used for creating large blocks of molten silicon. These are less effective in comparison with the former. Ribbon silicon is the subdivision of multi-crystalline silicon, and they are less efficient in comparison with polycrystalline structures.
Regardless of the materials used, the principle of getting electric energy stays the same. Solar cells use the principle of the p-n junction when the special changes are used in the molecules of monocrystalline semiconductors. This is generally achieved by alloying or diffusing semiconducting crystalline structures with a different semiconducting material together.
Conclusion
Solar energy is regarded as the key alternative for future energy consumption, as the potentials of photovoltaic technology development are endless. In general photovoltaic panels are used not only in households but also in industry and scientific aims. The sphere of application may be extended essentially.
However, the costs and effectiveness of the contemporary crystalline junctions leave much to be desired. The effectiveness of the technology is in inverse proportion to its costs. However, the actual effectiveness of the solar cells is sufficient for using them as an alternative source of energy for various aims.
Solar cells are highly dependent on their spatial position as well as the temperature of the environment, as the ideality factor of solar elements is closely related to the matters of reaction of the material for sunlight radiation as well as the temperature of the environment. The alternatives of photovoltaic elements development are closely linked to studying the molecular and atomic physics of silicon structure, as this stays the only element suitable for creating photovoltaic elements.
Reference List
Goetzberger, A., Hoffmann, V.U. 2005, Photovoltaic Solar Energy Generation, Springer
Lynn, P. A., 2010 Electricity from sunlight: An introduction to photovoltaics, Wiley-Blackwell
Pachauri, R. K., Abeyratne, S., 2000 From sunlight to electricity (Solar photovoltaic applications), University of New South Wales Press