Introduction
Aspirin is the common name for acetylsalicylic acid. Acetylcyclic acid is commonly used to control fever and as a pain killer. Salicylic acid is administered in the form of tablets, a less irritating method, unlike in the early days where it was administered in the form of leaves (Jeffreys, 2005). Irritation caused by leaves was due to the presence of a phenol group that is known to cause irritation. Aspirin is also used in low dosages to prevent diseases such as heart attack and stroke. Further, aspirin is useful in the treatment of colorectal cancer.
However, usage of aspirin has got side effects. Among the side effects include; ringing in the ears and stomach bleeding. According to Jeffreys (2005), aspirin is used in the treatment of rheumatic fever, inflammatory diseases such as arthritis and fever pain. Jeffreys (2005) describes aspirin as white, weakly acidic substance and crystalline in nature. The product has a melting point of 136 degrees Celsius and has a boiling point of 140 degrees Celsius.
According to McMurry (2008), aspirin exhibits polymorphism. Polymorphism is a process whereby a substance exists in more than one form. Before 1960s, aspirin was known to have only one structure. However, in 2005, Vishweshwar and coworkers discovered a second structure of aspirin. The second structure is stable at 100K and a change in temperature reverts it to structure one (McMurry, 2008). For drugs to receive regulatory approval, they must have only one crystal form. Therefore, pharmacists developed methods of ensuring that aspirin exhibits only one crystalline form.
The manufacturing process of aspirin is an esterification type of reaction (Pavia, 2005). Esterification process was invented after the Bayer Company of Germany replaced the phenol group in aspirin with an ester group. The ester group was less irritating although it could cause hemorrhaging. During the preparation process, salicylic acid is reacted with excess acetic anhydride (Pavia, 2005). A little amount of phosphoric acid is used as a catalyst to speed up the reaction. Then, the excess acetic acid is quenched with water. Due to the insoluble nature of aspirin, the compound precipitates on addition of water. The chemical equation for the reaction is as shown in the scheme below.
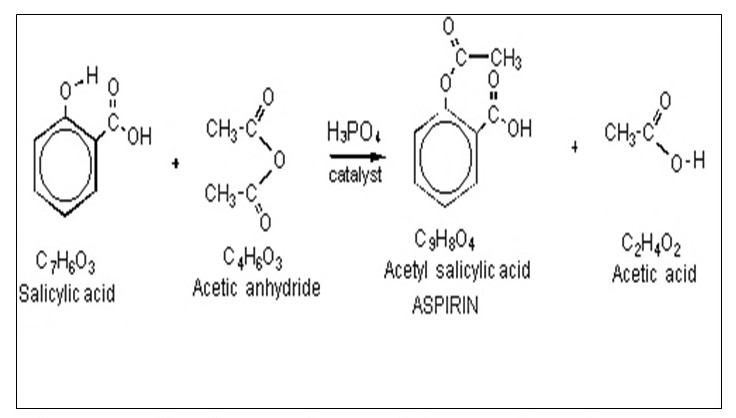
Diagram 1 showing a scheme of the reaction when aspirin reacts with water.
Acetic acid is highly soluble in water and, therefore, is separated with ease from the aspirin product. The product obtained at this stage is a crude product and, therefore, has to be purified. The purification process entails recrystallization of the crude product obtained in hot ethanol. However, the crude product is the desired result during the synthesis of aspirin. The analysis involves determining the percentage yield of the crude product and analyzing the purity of the product (Pavia, 2005). The purity of the product is examined using three methods: titration, melting point, and spectroscopic assay.
For a pure aspirin, the melting point is 138-140 degrees Celsius. The melting point of salicylic acid is 158-161 degrees Celsius. Impurities lower the melting point and can also increase the melting point by two degrees. Using titration, the acetylsalicylic moles are determined by assuming that the content of acid impurities is little in the crude sample (Pavia, 2005). The spectroscopic analysis entails the reaction between complex iron(III) with a deprotonated form of salicylate ion to form a purple solution. The aspirin product obtained in the reaction and is compared to standardized 0.15% ferric-salicylate solution. According to McMurry (2008), aspirin decomposes in absence of moisture into salicylic acid and acetic acid. The decomposition of aspirin is a reverse reaction of the synthesis reaction. However, the amount of free salicylic acid in an aspirin sample should not exceed 0.15% salicylic acid.
The experiment seeks to investigate the process of synthesis of aspirin. The experiment used acetic anhydride and salicylic acid to monitor the process. Further, the experiment aims to examine the purification process of aspirin using the melting point method.
Methodology
In this experiment, a 600 ml beaker containing about 450 ml of water was warmed to around 75 degrees Celsius. The flask was then clamped and heated for another 30m minutes. The temperature of the water was maintained at 75 degrees through the entire process. Then, 2ml of deionized water was added to the flask. Exactly 2.0 grams of salicylic acid was then transferred into a 50ml Erlenmeyer flask. 5.0 ml of acetic anhydride was added to the flask in a manner that washed crystals on the walls of the flask. Five drops of 85% phosphoric acid were then added. However, caution was taken in handling the phosphoric acid to avoid chemical burns. After vaporization, the flask was cooled for five minutes for crystals to form. The aspirin crystals were collected by filtering the liquid through Millipore funnel. Then the filtrate was heated at 45 degrees for ten minutes and later put in a vacuum for ten minutes. The crystals in the petri dish were then weighed. The heating process was repeated until a constant weight of crystals was recorded. The crystals were then stored for purity testing.
Results
The table 1 below shows the results obtained from the experiment. The entire results were summarized in a single table for easy analysis.
Table 1
Table 1 showing a summary of results obtained from the experiment.
The theoretical melting point of aspirin is 136oC. As such, the end product is actually aspirin since its melting point (136.1oC) is approximately equal to the literature value. In a synopsis, the experiment was a success since there were negligible impurities in the final product.
Esterification is the process that results in the formation of aspirin. In this process, the reactants include an alcohol and a carboxylic acid that result in the formation of an ester as the end product. With regards to the production of aspirin, the reactants include the salicylic acid and the acetic acid. The reaction is executed in presence of a catalyst, sulfuric acid, under high temperature condition. Whatever happens within the reacting molecules is that water dissociates from the carboxylic acid, consequently the resulting fragment merges with those from the alcohol to for an ester called acetylsalicylic acid otherwise aspirin. The reaction is shown in the figure 1 above.
In this experiment, phosphoric acid was used instead of sulfuric acid. Either of the two acids can be used as a catalyst to speed up the esterification reaction.
In this experiment the yield can be calculated from the equation below:
% yield = (product mass)/(mass of the raw material used or salicylic acid).
Therefore, %yield = 1.4311/1.9994 x 100%
= 71.58 ± error%
But error = (ymax-ymin)/2 = ±0.25%.
= 71.58 ± 0.25%.
Discussion
In the laboratory, the main objective was to investigate the process of synthesis of aspirin. The experiment used acetic anhydride and salicylic acid to monitor the process. Further, the experiment examined the purification process of aspirin using the melting point method, and later the yield was determined. From the experimental results, the objectives of the experiment were met, indicating that aspirin is a product of the esterification process between salicylic acid and the acetic acid. The purity of the product informed my aforementioned assertion. As such, the purity (melting point of 136.1oC) was found to be approximately equal to the theoretical value (136oC). From the results obtained it can be concluded that the experiment was a near-perfect one with negligible errors. However, the yield (71.58 ± 0.25%.) of the experiment was not perfect, but it was good since the experiment’s objective was to test the effectiveness of the process with respect to quality. Nonetheless, for commercial purposes all the loopholes for product losses ought to be sealed. This would result in a higher yield than what the experiment gave.
The esterification process that yields aspirin is a slow process thus there it was important to incorporate a catalyst in the reaction speed up the reaction. The catalyst action is to lower the activation energy or the energy ‘hill’ to allow the reactions to be executed using less energy. The catalyst used in this experiment is appropriately chosen; the one with the ability to accept lone pair electrons. To this end, phosphoric acid served the purpose perfectly.
The purification of the product could also be confirmed by other methods just to ensure that the experiment was effective with respect to purity. Alternative methods for checking the purity of the product include testing the solubility of the product as well as using infrared spectra. The aforementioned methods are some of the physical tests that can be done to determine the product’s purity. Moreover, there are other chemical methods that include phenol test and potentiometric titration. The physical tests are more reliable since the chance of introducing an extraneous element is minimal.
Importantly, in this experiment the purification of the product was achieved via recrystallization. This method is usually rapid, and the process occurs when the impure material and a solvent that is hot is mixed and then cooled gradually. As the mixture cools, it becomes saturated, forming the crystals of the product. In the process, the impurities are left in the solution.
Conclusion
In a conclusion, the objective of this experiment was met by testing the purity of the end product (136.1oC) that resulted from the process. The percentage yield was also established to be 71.58 ± 0.25%.
References
Jeffreys, D. (2005). Aspirin: The remarkable story of a wonder drug. London: Idea Group Publishing.
McMurry, R.S. (2008). Organic Chemistry. New York, NY: Norton & Company.
Pavia, D. L. (2005). Introduction to organic laboratory techniques: A small-scale approach. London: Sage Publications.