Topic: Type II Diabetes Mellitus
- Patient: a 47-year-old female with a three-year-old history of Type II DM.
- It is her third visit to a hospital.
- Chief complaints: weakness during the last five days and frequent urination.
In this presentation, we are going to develop a care plan for a 47-year-old woman with a 3-year-old history of Diabetes Mellitus Type 2 (also known as Type II DM). Type II DM (ICD-10-CM: E11.9) is one of the common public health problems caused by a failed production of insulin and an insufficient production of beta-cell receptors (Coelho et al., 2016). It is a chronic condition in which the body metabolizes a high level of glucose (sugar) (Pradeep & Haranath, 2014). The patient visits a hospital for the third time with such symptoms as weakness during the last five days. She observes that her urination has become more frequent. Frequent urination and weakness are not the only symptoms that prove the development of this disorder. It is also necessary to focus on such changes as slow-healing sores, weight loss, increased hunger, and the color of skin.

Pathogenesis
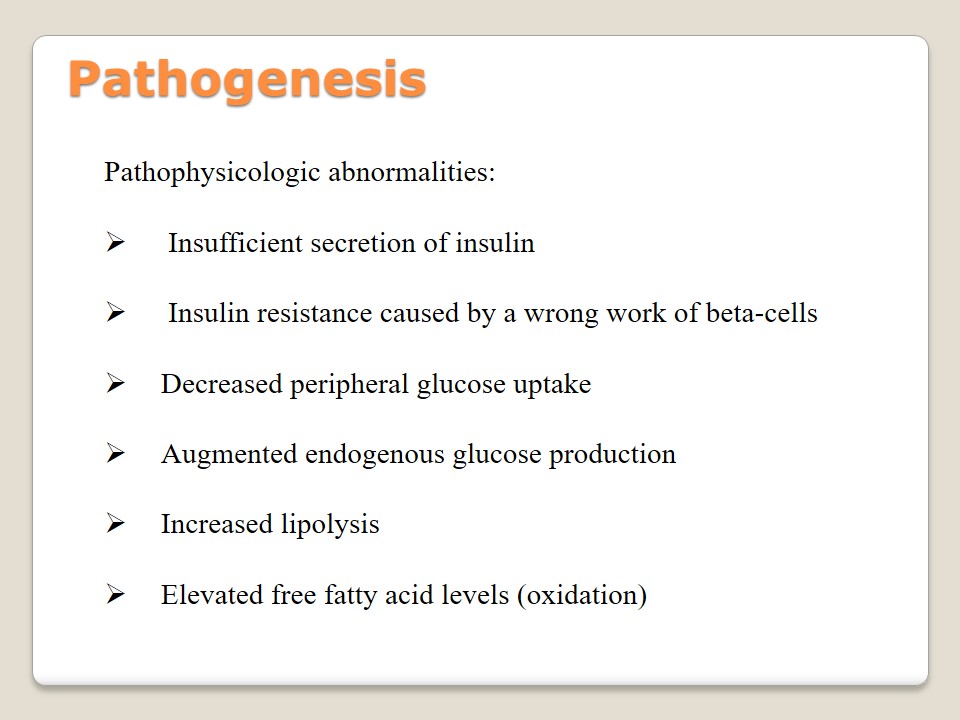
Pathophysicologic abnormalities:
- Insufficient secretion of insulin.
- Insulin resistance caused by a wrong work of beta-cells.
- Decreased peripheral glucose uptake.
- Augmented endogenous glucose production.
- Increased lipolysis.
- Elevated free fatty acid levels (oxidation).
Type II diabetes is caused by insufficient secretion of insulin that is produced by beta-cells or insulin resistance that results in the inability of cells to respond to the insulin hormone (Pradeep & Haranath, 2014).
It is possible to define several important abnormalities to explain the pathogenesis of the chosen disease, including (Cersosimo, Tiplitt, Mandarino, & deFronzo, 2015):
- Insufficient secretion of insulin is connected with tissue requirements in terms of which the level of blood glucose may be controlled;
- Insulin resistance caused by a wrong work of beta-cells – insulin secretion is usually regulated by glucose metabolism, and the work of beta-cells defines the quality of this process;
- Decreased peripheral glucose uptake – this change influences the work of glucose transporters which are connected with skeletal muscle and adipose tissue;
- Augmented endogenous glucose production – this processes has to be controlled to report on any possible deviation in the body;
- Increased lipolysis – this condition includes a breakdown of stored fat that can lead to unpleasant chemical reactions in the body;
- Elevated free fatty acid levels (oxidation) proves insulin resistance and causes endothelial dysfunction.
Epidemiology
In 2014, more than 422 million of a global population have Type II DM.
1.6 million people died because of diabetes.
Prevalence of diabetes among adults has increased to 8.5% and among children – up to 2.8%.
Diabetes leads to blindness, heart attacks, and strokes.
People from low- or middle-income families are under a threat.
According to the investigations of the World Health Organization (2017), the number of people with diabetes has considerably increased during the last decade. For example, at the end of the 1900s, only 108 million people with diabetes were recorded, and at the beginning of the 2000s, approximately 422 million people have this disease globally. Besides, about 1.6 million deaths are caused by diabetes. Regarding Type II diabetes patients, 8.5% of them are adults, and 2.8% of them are children or adolescents (Reinehr, 2013; World Health Organization, 2017). Diabetes is a serious disease that may be characterized by different complications, the most dangerous of which are blindness, heart attacks, and strokes. The World Health Organization (2017) states that people from middle- and low-income families are exposed to having diabetes more frequently than rich people.
Clinical Presentation: History
- Hypertension history – 15 years.
- Considerable weight loss at the age of 27.
- Smoking history (two packs per week) – 2o years.
- Alcohol – 1-2 glasses of wine on weekends.
- No drugs, no allergies, no surgeries.
- Noticeable weakness during the last five days.
- Frequent urination even if she drinks or eats nothing.
- Red flags: obesity, weight loss, and frequent urination.
When the patient comes to a hospital, she has to share her medical history. Her hypertension history began 15 years ago. At the age of 27, she tried several diets to deal with her several extra pounds. She lost about 15 kg and continued doing physical exercises regularly. She has been smoking for the last 20 years. She finds that it is normal to smoke 1-2 packs per week and does not find it necessary to quit smoking. Her alcohol usage is about 1-2 glasses on Sunday evening with a company of her close friends. She denies the usage of drugs. No surgical history. No allergies. Her last observations include the fact that she feels fatigue and weakness even if she does not take hard exercises. Besides, she goes to bathroom frequently, even if she does not drink or eat at the moment or before.

Clinical Presentation: Physical Exam
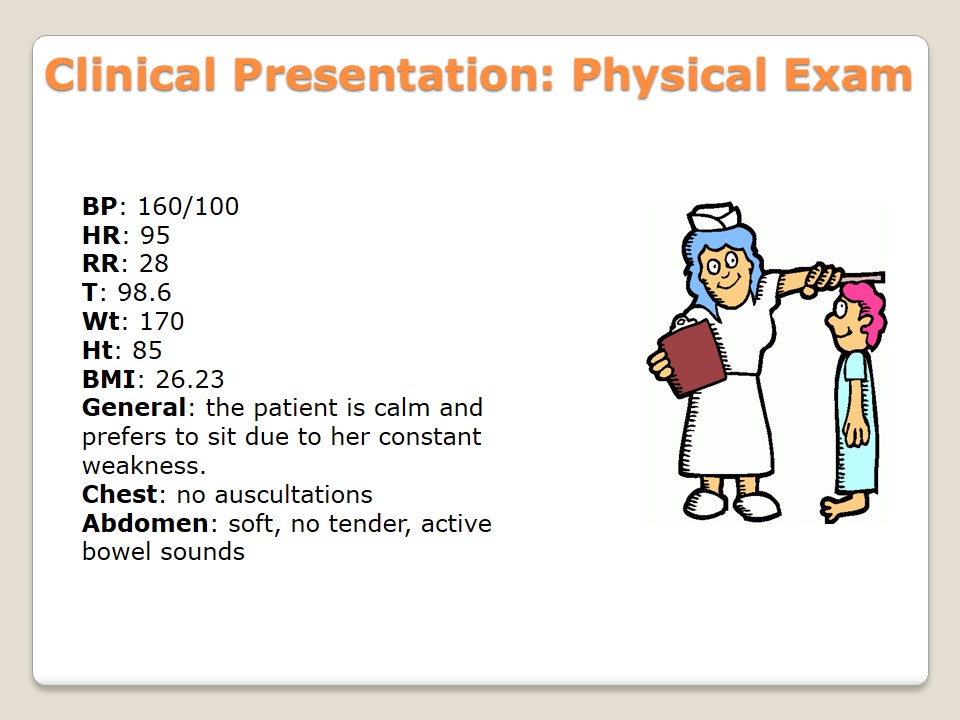
- BP: 160/100;
- HR: 95;
- RR: 28;
- T: 98.6;
- Wt: 170;
- Ht: 85;
- BMI: 26.23;
- General: the patient is calm and prefers to sit due to her constant weakness.
- Chest: no auscultations.
- Abdomen: soft, no tender, active bowel sounds.
During a physical examination of a patient, the vital signs have to be mentioned. These clinical findings help to obtain a clear picture of a patient and investigate if they are normal or not. Blood pressure is 160/100. This sign proves chronic hypertension of the patient. Pulse is 95 beats per minute. It is a norm for women. Still, it is close to a high level. Respiration rate is 20 beats per second. This number is defined as high. Finally, the patient’s BMI is 26.23. It is a sign of overweighing. Her weight is 170 cm, and her height is 85 cm. Though she does some physical exercises, this category of overweight is the lowest and can be fixed in case certain measures are taken.
Differential Diagnoses
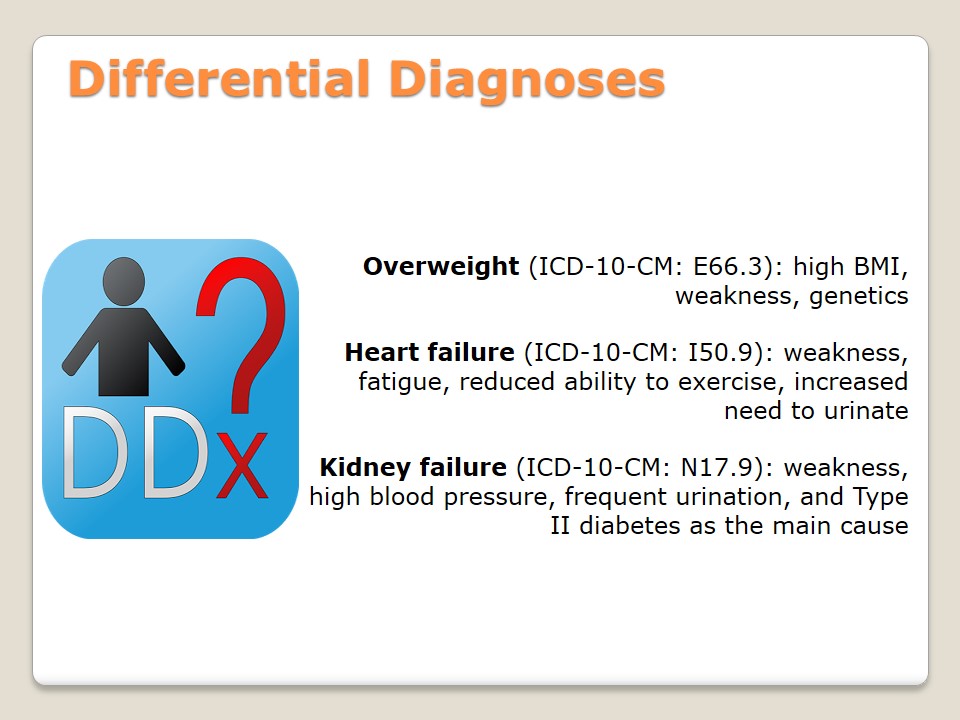
- Overweight (ICD-10-CM: E66.3): high BMI, weakness, genetics.
- Heart failure (ICD-10-CM: I50.9): weakness, fatigue, reduced ability to exercise, increased need to urinate.
- Kidney failure (ICD-10-CM: N17.9): weakness, high blood pressure, frequent urination, and Type II diabetes as the main cause.
Three possible differential diagnoses can be given to the patient regarding her main complaints, including weakness, frequent urination, and other associated complications like the impossibility to exercise frequently and confusion due to fatigue.
- Overweight is a complex disorder that is usually characterized by an excessive body fat. Overweight is a risk factor for women with diabetes (Ollila et al., 2017). The number of health problems may be considerably increased due to overweight.
- Heart failure is a condition when a heart cannot pump the required amount of blood. Type II DM increases the risk of having a heart failure in women older than 40 years (Zannad et al., 2015). Glucose-lowering medications may lead to a serious cardiovascular harm.
- Kidney failure is a condition when kidneys are not able to control waste products obtained from blood. Blood chemicals may be dangerous for the body, and Type II diabetes is a disease that is characterized by such complications as kidney failure (American Diabetes Association, 2015). It is necessary to optimize glucose control and stabilize blood pressure to avoid the development of further complications.
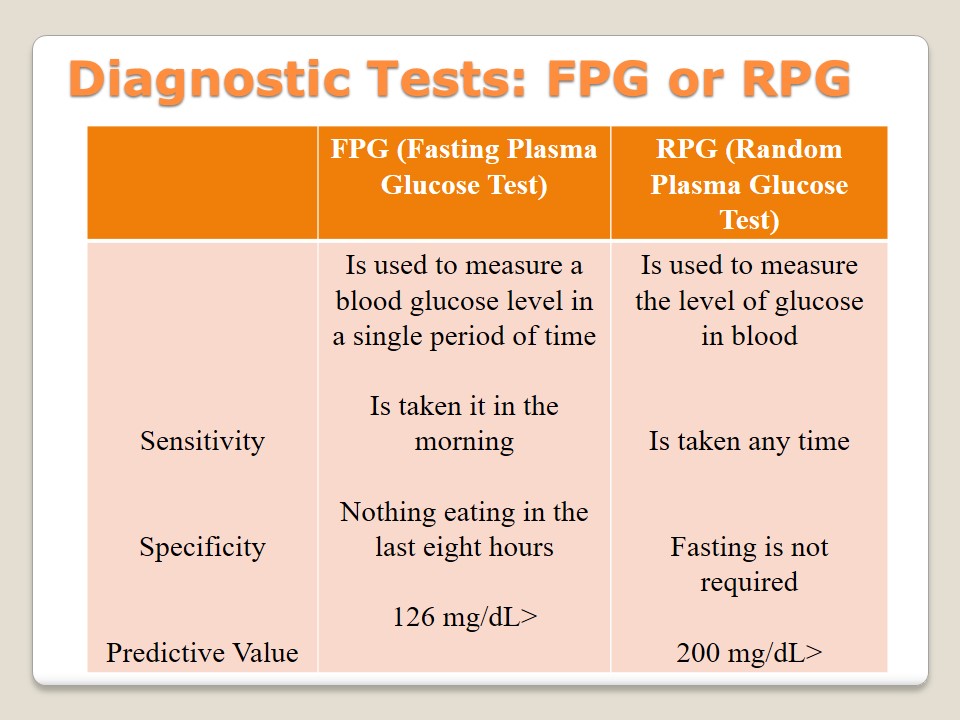
Diagnostic Tests: FPG or RPG
To identify the category of diabetes, it is suggested to take plasma glucose tests. There are two types of tests available to patients. Both of them aim at measuring the level of glucose in blood. First, there is a Fasting Plasma Glucose test that has to be taken in the morning as a result of fasting (meaning not eating during the last eight hours). If the level of sugar is 126 mg/dL and more, a patient has diabetes (American Diabetes Association, 2015). Second, there is a Randon Plasma Glucose test with the help of which it is possible to measure the level of sugar any time because fasting is not required. It is used in case urination becomes more frequent and other symptoms are observed.
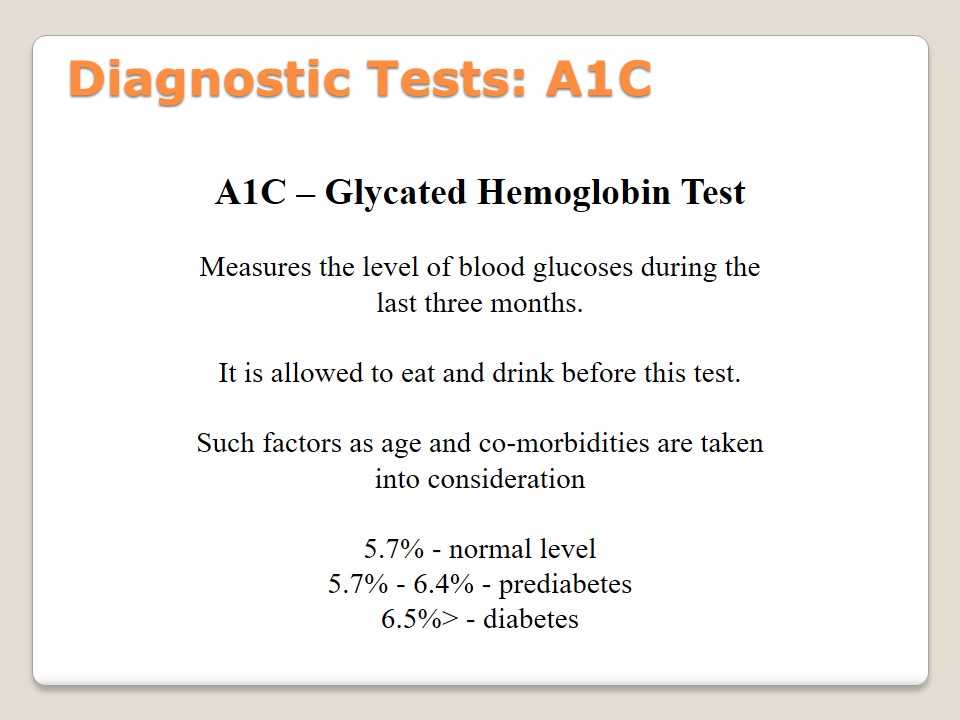
Diagnostic Tests: A1C
A1C – Glycated Hemoglobin Test:
Measures the level of blood glucoses during the last three months.
It is allowed to eat and drink before this test.
Such factors as age and co-morbidities are taken into consideration.
- 5.7% – normal level;
- 5.7% – 6.4% – prediabetes;
- 6.5%> – diabetes.
Glycated hemoglobin test, also known as A1C or HbA1C, is used to indicate an average level of glucose in blood in the period of the last three months. The peculiar feature of this test is the possibility to identify an average level of sugar during the last three months. There are no measurements that make this test sensitive because it is allowed to eat and drink anytime. The specificity of A1C is the consideration of age and different co-morbidities of a patient. A predictive level in this test is as follows: 5.7% – normal, 5.7% – 6.4% – prediabetes, 6.5%> – diabetes (American Diabetes Association, 2015).
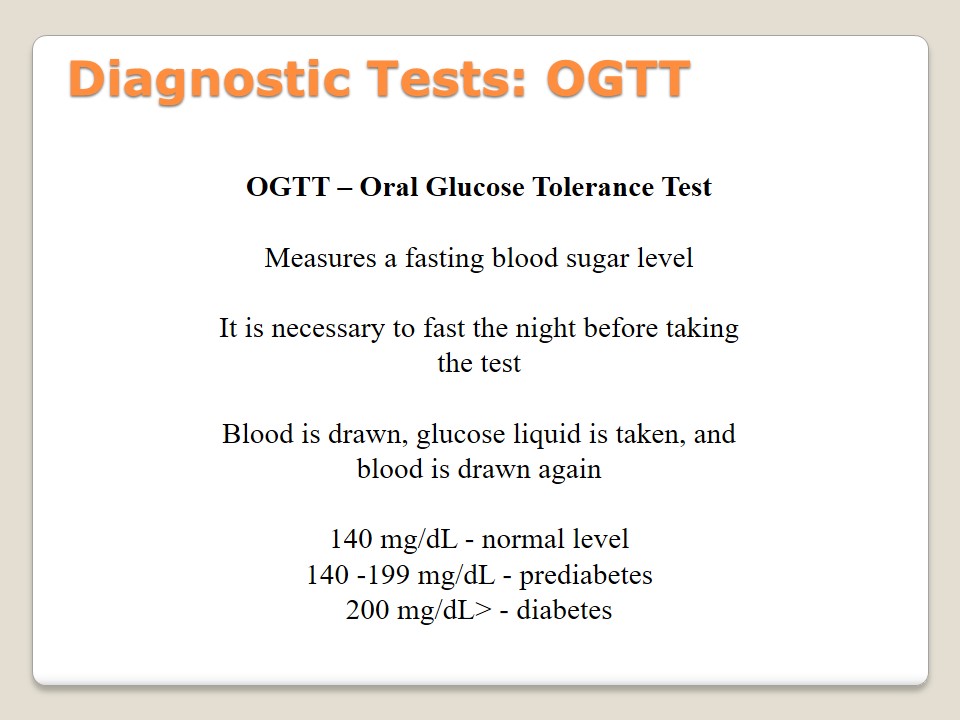
Diagnostic Tests: OGTT
OGTT – Oral Glucose Tolerance Test:
Measures a fasting blood sugar level.
It is necessary to fast the night before taking the test.
Blood is drawn, glucose liquid is taken, and blood is drawn again.
- 140 mg/dL – normal level;
- 140 -199 mg/dL – prediabetes;
- 200 mg/dL> – diabetes.
Oral Glucose Tolerance test is taken by the patients who have to measure their sugar level. This test should be repeated annually to understand if the level of sugar changes, and if a new treatment approach is required. Patients should fast at least 8 hours before taking OGTT. Its specificity is the necessity to draw blood before and after taking specific glucose liquid. Then, the analysis of the results should be developed. According to American Diabetes Association (2015), the norms are following: 140 mg/dL (normal), 140 -199 mg/dL (prediabetes), 200 mg/dL> (diabetes). If FGP or RPG tests have already been taken, OGTT is not necessary (Pradeep & Haranath, 2014).
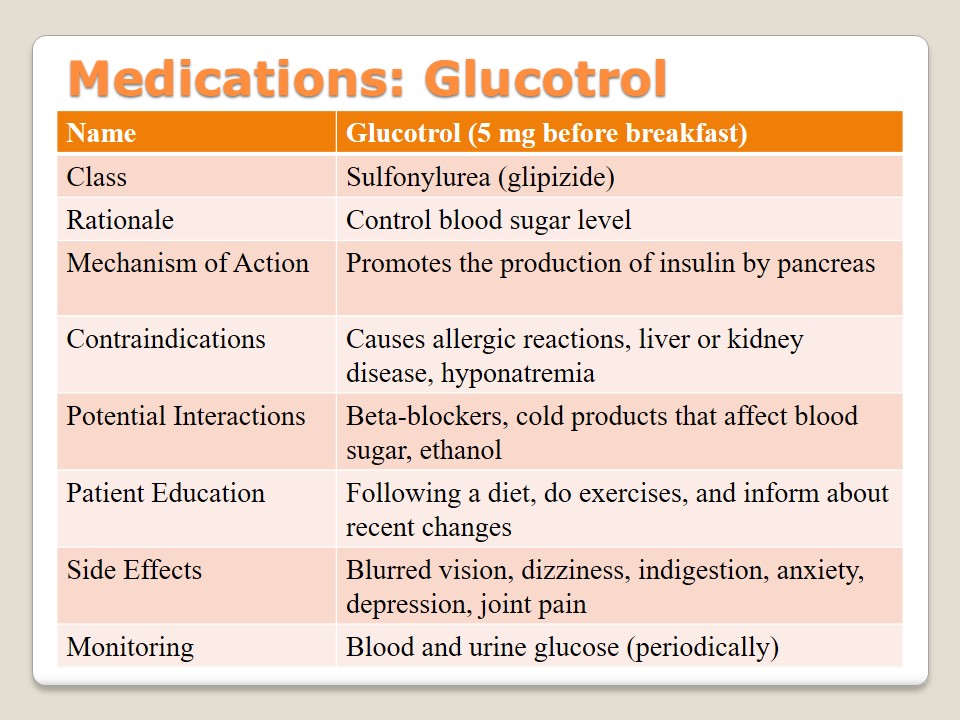
Medications: Glucotrol
Sulfonylurea is a class of medications which include such types as “tolbutamide, glibenclamide, chlorpropamide, glipizide, acetohexamide, gliclazide and tolazamide” (Pradeep & Haranath, 2014, p. 26). Glucotrol is a medication of this class that belongs to a group of glipizide which helps to control the level of blood sugar. The patient should take it before breakfast (5 mg). This medication is responsible for the improvement of pancreas and its insulin production. The patient should know about contraindications such as allergies, problems with liver or kidneys, or hyponatremia. Glucotrol interactions with beta-blockers, cold products, and ethanol have to be observed carefully. Besides, the patient should follow a diet, do physical exercises, and inform a doctor about any changes in the body due to the possibility of such side effects as blurred vision, dizziness, depression, or joint pain. Blood and urine glucose levels have to be periodically monitored.
Medications: Glumetza
Glumetza is a drug from the Metformin class that is used to control the level of blood sugar. It is usually suggested to obese people because it helps to improve glucose tolerance and lower plasma glucose. Patients have to be careful with contraindications such as allergies, renal dysfunction, and problems with breathing or blood. A certain attention should be paid to interactions with such drugs as beta-blockers, cationic drugs, Glyburide, and Furosemide. Patients should never share this medication with other people and be careful not to overdose. If such side effects as sweating, dizziness, or hunger are observed, it is necessary to address a doctor. Lactic acidosis is a dangerous outcome. Renal function, as well as the level of blood glucose, has to be regularly monitored.
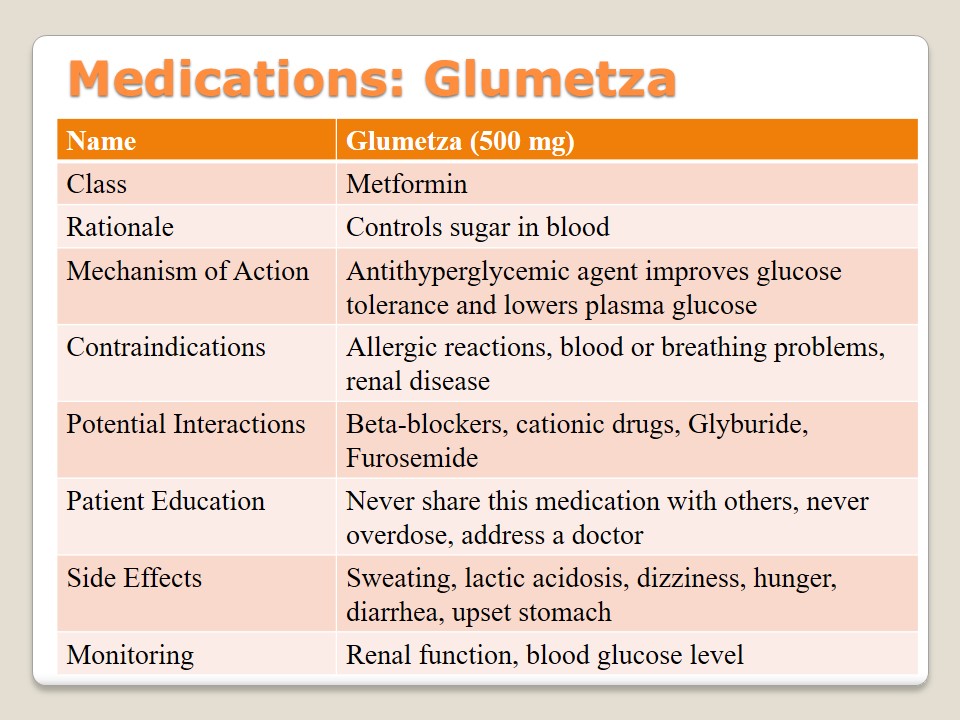
Medications: Acarbose
Acarbose is a medication of an alpha-glucosidase inhibitor class the goal of which is to deal with hyperglycemia patients and promote blood-glucose concentration. Its mechanism of action is closely connected with the work of enzymes and their effects on carbohydrates in the body. If people suffer from diabetic ketoacidosis, bowel disease, or chronic intestinal disease should consult their physicians before taking this drug. Besides, the interactions with such substances as thiazides, estrogen, and phenothiazine or some oral contraceptives. Patient education includes the discussions about possible overdoses, the necessity to control the level of glucose in blood, and its main side effects (diarrhea, flatulence, or abdominal pain). Monitoring of blood glucose is required.
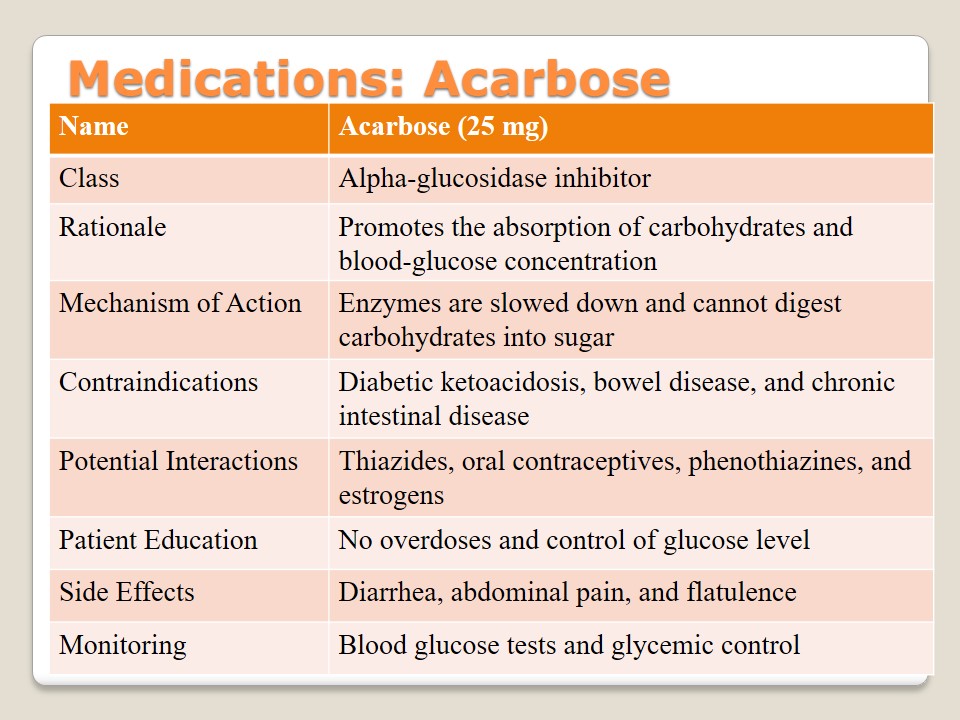
Medications: Insulin
Insulin is a form of medication that is used to treat diabetes and provide patients with a hormone with the help of which it is possible to stabilize the necessary level of blood sugar. It is used as injection. Patients with allergies and hypoglycemia should consult their doctors before taking it. Education about its interactions with beta blockers, alcohol, ACE inhibitors, and clonidine is required to prevent the dysfunction of lungs. The main side effects are pruritus, edema, rash, and hunger. Allergies to some of the insulin components may be observed. Body weight and blood glucose have to be regularly monitored.
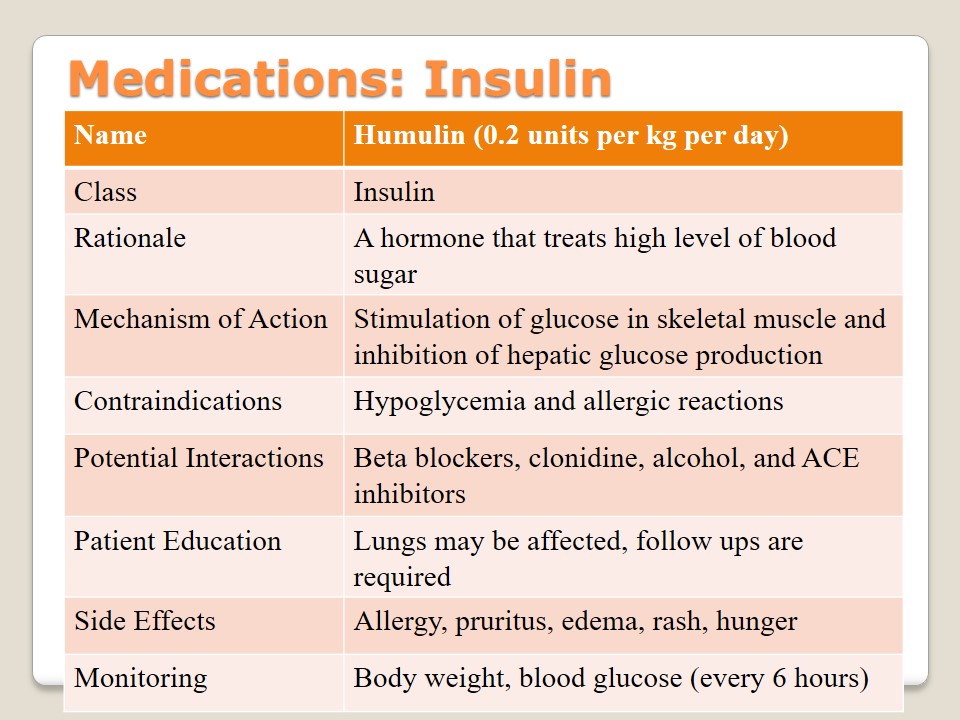
Medications: Actos
Actos is a brand name of the medications of pioglitazone class the goal of which is to lower the level of sugar in blood. This antidiabetic agent aims at decreasing insulin resistance and increasing glucose disposal. Such conditions as pregnancy, hepatic impairment, bladder cancer, and macroscopic haematuria should be defined as the main contraindications of the drug. The patient should learn about possible allergies caused by Actos and the nature of interactions with different oral contraceptives, metformin, glipizide, and warfarin. Change of weight, overdose, blurred vision, and problems with hearing or livers should be identified.
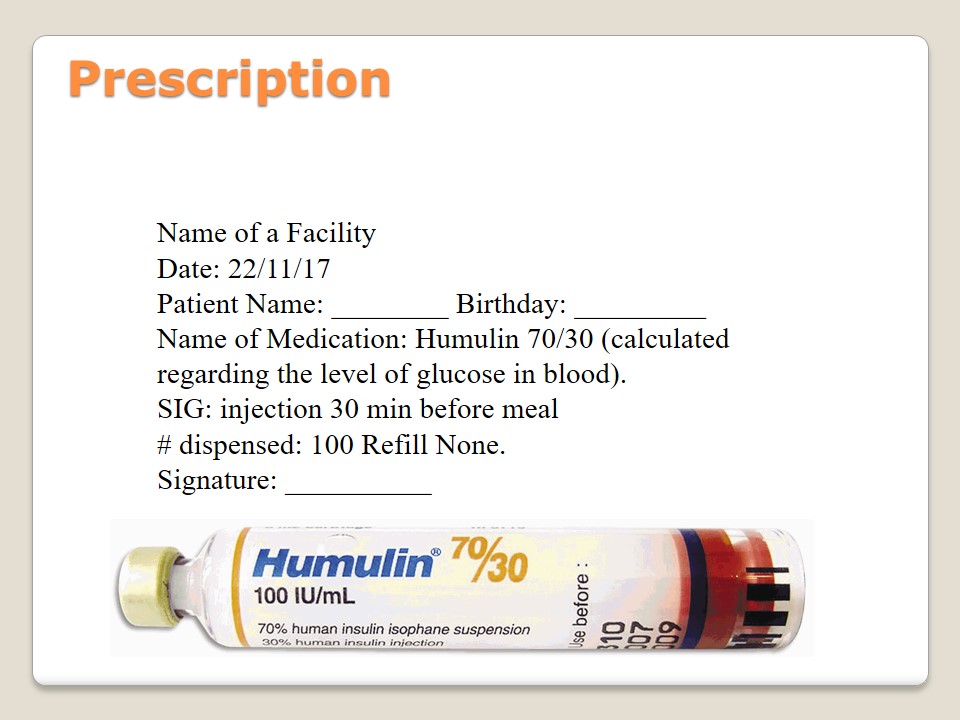
Prescription
- Name of a Facility
- Date: 22/11/17
- Patient Name: ________ Birthday: _________
- Name of Medication: Humulin 70/30 (calculated regarding the level of glucose in blood).
- SIG: injection 30 min before meal
- # dispensed: 100 Refill None.
- Signature: __________.
All above-mentioned medication require prescriptions that have to be given by a doctor. It is hard to find a place where it is possible to buy some diabetes drugs without prescriptions. Besides, all reliable associations and well-known organizations suggest not buying diabetes drugs which are over-the-counter. It is unsafe and may lead to a number of negative outcomes. Still, if a person is in need of some medication at the moment, it is possible to pay attention to the places where insulin can be sold without a prescription.
Care Plan
- Follow-Up:
- regular meetings with a primary health care provider;
- annual tests;
- Referrals:
- podiatrist;
- psychologist;
- Education:
- what to expect;
- why to follow a diet;
- what lifestyle to follow;
- how to encourage physical activities;
- Care:
- blood pressure control;
- dietary issues;
- vaccination.
It is important for a patient never omit meetings with a primary healthcare provider and take all necessary test to control the level of glucose or indicate other body changes. It is possible to address such specialists as podiatrists or psychologists in case certain problems bother a patient. Education should cover different aspects of routine life, including expectations, diets, lifestyle, and physical activities. A certain attention should be paid to blood pressure control, dietary issues, and vaccination.
References
American Diabetes Association. (2015). Standards of medical care in diabetes—2015 abridged for primary care providers. Clinical Diabetes: A Publication of the American Diabetes Association, 33(2), 97-111.
Cersosimo, E.,Tiplitt, C., Mandarino, L.G., & deFronzo, R.A. (2015). Pathogenesis of type II diabetes mellitus. Web.
Coelho, G. D. P., Martins, V. S., Amaral, L. V. D., Novaes, R. D., Sarandy, M. M., & Gonçalves, R. V. (2016). Applicability of isolates and fractions of plant extracts in murine models in type II diabetes: A systematic review. Evidence-Based Complementary and Alternative Medicine, 2016. Web.
Ollila, M. M., West, S., Keinänen-Kiukaanniemi, S., Jokelainen, J., Auvinen, J., Puukka, K., … Piltonen, T. T. (2017). Overweight and obese but not normal weight women with PCOS are at increased risk of Type 2 diabetes mellitus—A prospective, population-based cohort study. Human Reproduction, 32(2), 423-431.
Pradeep, T., & Haranath, C. (2014). A review on diabetes mellitus type II. International Journal of Pharma Research & Review, 3(9), 23-29.
Reinehr, T. (2013). Type 2 diabetes mellitus in children and adolescents. World Journal of Diabetes, 4(6), 270-281.
World Health Organization. (2017). Diabetes. Web.
Zannad, F., Cannon, C. P., Cushman, W. C., Bakris, G. L., Menon, V., Perez, A. T., … Lam, H. (2015). Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: A multicentre, randomised, double-blind trial. The Lancet, 385(9982), 2067-2076.