Introduction
Nearly every element of within the periodic table has found its way into the atmosphere. As an ease when studying the element and there species composition of compounds in the atmosphere, Seinsfield and Pandis (21) suggest categorizing of the atmospheric compounds as containing halogens, sulfur, carbon or nitrogen.
Probably, this classification rides on the fact that emissions into the atmosphere breakdown from original compounds into component species before exiting the atmosphere in a cyclic phenomenon. Actually, the cyclic process of substances is contained in the biogeochemical cycle of elements.
The scope of understanding the cyclic processes comprises atmospheric movement across Oceania, land terrains, biospheres, inter alia; chemical transitions (quantity and quality) of the substances and rates of circulation and transfer (Seinsfield and Pandis 21).
The geographical paradigm gives this subject the spatial and temporal references, since the transportation aspect is a vector quantity that can be justified through a scalar quantity of time and direction.
Mehta (124) explains that in 1852, Robert Angus Smith made-up the term acid rain. It was not until 1972, when the concept of acid rain became familiar in the western industrial world. The term referred to atmospheric acidity levels (at pH above 5.6) that surpass normal levels for rain, fog and smog.
The precipitation (deposition) of these acidic concentrates impacted on ecosystems, antiquities and human health. Acid rain was traced back into the gradual Geo-biological processes within nature and accelerated volcanic emissions (Mehta 124).
The problematic scope of acid deposition gained wider magnitude when it was realized that it evolved into a trans-boundary affair. It was revealed that there was mobility of precursor elements emitted from the industrial heartlands in Europe and North America.
Emissions containing precursors- sulfur dioxide and oxides of nitrogen (referred to as NOX species) form the major bulk of acid rain. In order to effect solutions towards acid rain, a critical loads framework on emission cuts and ecosystem recovery was developed. Already, Europe has generated maps depicting critical loads.
Driscoll, Lambert and Chen (28) note that in the US three forms of acid deposition have occurred. These are wet, dry and cloud or fog depositions. Through research, more than 200 sites have been monitored as experiencing wet deposition consisting of rain, snow, sleet and hail.
Dry deposition consists of vapor, particles and gases. Some coastal areas and high altitudes have been exposed to dry and cloud deposition. Driscoll, Lambert and Chen (28) explain that the pattern of dry and cloud deposition widely varies spatially and temporally; thus, making it intricate to give consistent characteristics.
Because dry and cloud deposition can accompany the other two deposition forms then researchers have resorted to bulk deposition measuring using open collector.
Literature Review
Tracking Acid Rain: The Case study of The Rust Belt, US
Case Background
The industrial heartlands of the US are located in the Rust Belt (Midwestern American). The Rust Belt extends into Canada within the Canadian Heartlands. EIR/LaRouche Youth Movement Economics Team (2006) describes the Rust Belt as covering Pennsylvania and New York (Western) this extends into Missouri.
A quarter of the US populace resides within the industrial heartland. Mair et al. (361) indicate that heartland is at the center of a major transplant corridor for automobile manufacturers from the Asian and European world.
These industrial plants were strategically located to give them a competitive advantage in production capacity and market supply. The interest of the Asian investors to set up automobile plants in the US was driven by the protectionist belief of cutting down their exports into America (Mair et al. 355).
Mair et al (354) explains that the conceptual approach of the automobile plants was “Just-in-Time” to imply that the target was mass production to meet the demand within an area and the adjacent environs of America. Not only did the heartlands host automobile plants but also other electrical utilities and metal plants.
While the metal plants are in the east; the automobile plants are in the west of the heartland. Heartlands greatly symbolize the economic capacity and industrial advancements achieved.
Figure 1: Map of Heartland of North America
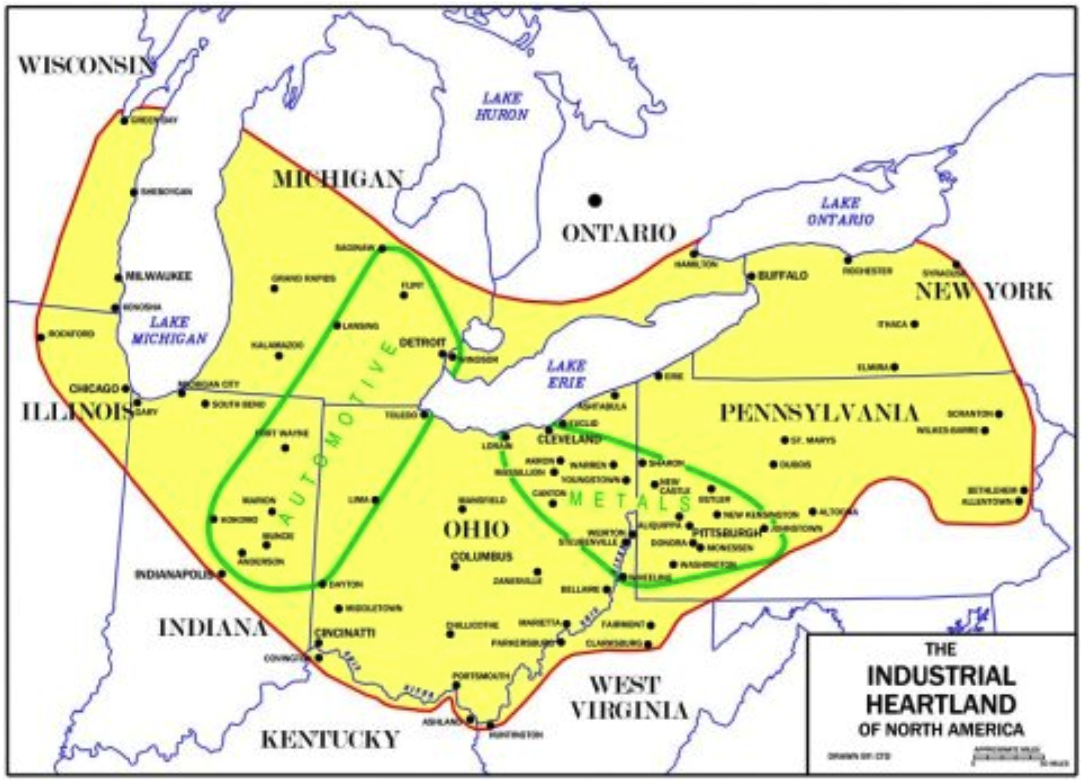
Source: “The Industrial Heartland of North America”
Lind (148) explains that long term rainfall data in the heartland area gave indications of acid rain. This has translated to heavy environmental damage. The extent of damage has contributed to a reduction in industrial development. This has translated into economic losses. In the far Northeast and outside the industrial heartland, the problem of acid rain continues to persist. Actually, about 33 percent of emissions causing acid rain in the down wind area (that is, far Northeast) traces back from the automobile sites in the Midwest (the source area). Lind (149) notes that economic factors have motivated the use of coal conversion and the combustion of sulfur containing coal. From an economic geography perspective, heartlands are tailored to receive raw inputs to facilitate industrial activities. Nevertheless, strategizing for the heartland location the risk regime and environmental impacts should be factored in. Lind (150) observes that effort to reach at a negotiated equitable solution between the source areas and the downwind areas have failed.
Factors Contributing to Acid Rain Formation
Acid rain has a set of preconditions that facilitate the formation process (Wang and Wang 2297). The concentration of the emitted precursor elements, compounds in rainfall, aerosols and their capacity to buffer and weather conditions are some of the contributory factors leading to acid formation.
Figure 2: Conceptual Framework to Acid Rain Formation
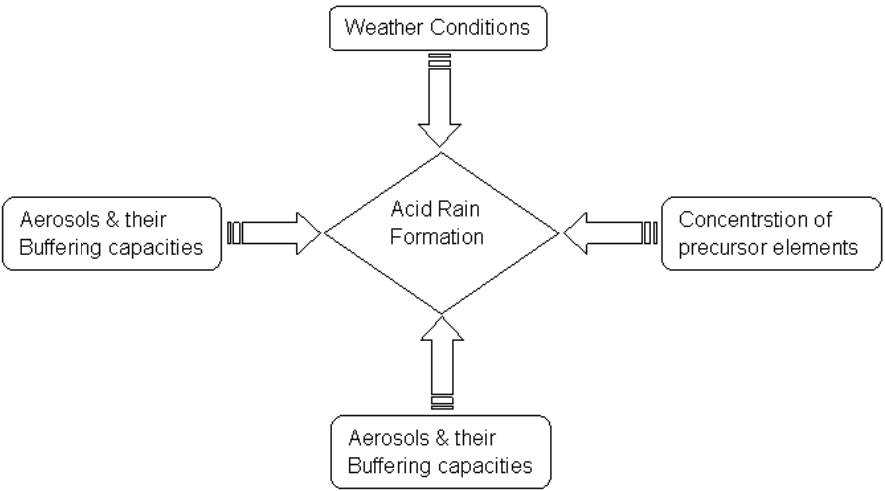
Figure 3: Acid Rain Cycle
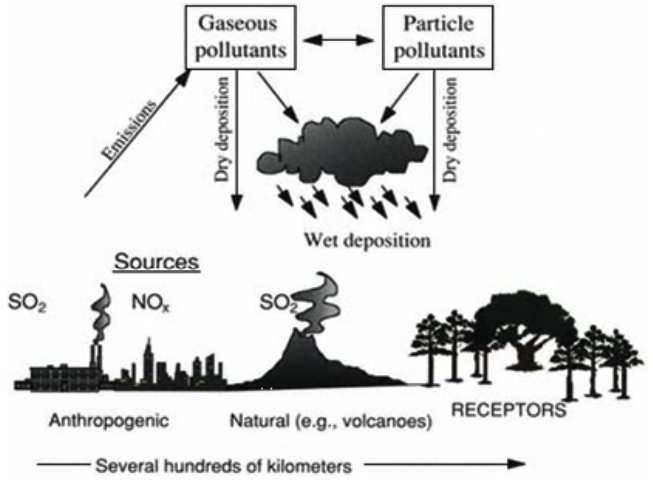
Source: Downing, Ramankutty and Shah (14)
Airborne pollution due to release of Sulfur dioxide and NOX species is the primary source of precursor elements leading to acid rain. Once in the atmosphere, these pollutants undergo a chemical interaction with atmospheric water and oxygen (Downing, Ramankutty and Shah 13).
In the presence of other atmospheric chemicals, sulfur dioxide and NOX species end up forming sulfuric acid and nitric acid. Emissions can remain within the atmosphere longer and a drift to far places prior to deposition on the surface. Prevailing winds play an important role in drifting the pollutants.
Deposition of acid rain takes many forms such as dew, snow, fog, so on (Downing, Ramankutty and Shah 13). Use of fossil fuel, sulfur containing coal and biomass combustion is the common sources of acid rain precursor elements.
Adverse effects of acid deposition include loss of forest cover through complex interactions, destruction of aquatic life and their ecosystems, loss of aesthetic value for monuments and cultural resources and human respiratory health risks Downing, Ramankutty and Shah 14).
Emissions are released from large point sources like combustion plants were thought to have a localized impact. Increased concerns based on this premise led to the building of new facilities that have longer smokestacks, tailored to disperse the emission over a wider area. Large scale dispersion and distribution of acidification may be a regional concern.
Acid Rain and Emission Cuts Milestone
In the United States, the proportional release by factory processes, electric utilities and combustions are two-thirds, 15 percent and 9 percent, respectively (Driscoll, Lambert and Chen 27). Moreover, automobiles account for over half of human related sources of nitrogen oxides.
Electric utilities and combustion processes account for 22 percent and 14 percent of NOX emissions, respectively. In 2002, more than 50 percent of precursor elements release occurred in seven states within the Ohio River Valley (Driscoll, Lambert and Chen 29).
Five of these states dominate in the release of nitrogen oxides. The decline of air quality forms an indicator of adverse impacts of release of precursor elements. In 1973, the level of emission in the United States had highs of over 29 million metric tons, yearly.
Within a period of twenty years since 1950 there has been a decline of over a half of sulfur dioxide due to the Amendments of the Clean Air Act (CAAA). In 2002, the emission levels were 13.9 million metric tons.
In 1990, NOX species emissions had the highest toll at 22.7 million metric tons. In the following decade, emissions declined by 12 percent. NOX emission targets were set to decrease by almost 2 million tons within the specifics of the 1990 CAAA (Driscoll, Lambert and Chen 27).
Moreover, there are state initiatives meant to augment emission cuts locally (Driscoll, Lambert and Chen 230). There have been international efforts towards emission cuts. The first treaty meant for emissions cut came into place in 1985.
The treaty was knowns as the Protocol on the Reduction of Sulfur Emissions. The emission cuts were set at 30 percent by 1993 vis-a-vis the 1980 levels (Driscoll, Lambert and Chen 30). Further treaties set the cuts at 80 percent with reference to 1980 levels.
Further treaties on emission cuts in the decade beginning in 1990; have led to declines of sulfur dioxide and NOX species at two-thirds and a third, respectively (Driscoll, Lambert and Chen 30). The LRTAP Protocol of 1999 introduced the concept of critical loads that led to the development of critical load maps within the European context.
Analysis
Figure 4: 1.0 Trends in Emission of Acid Rain
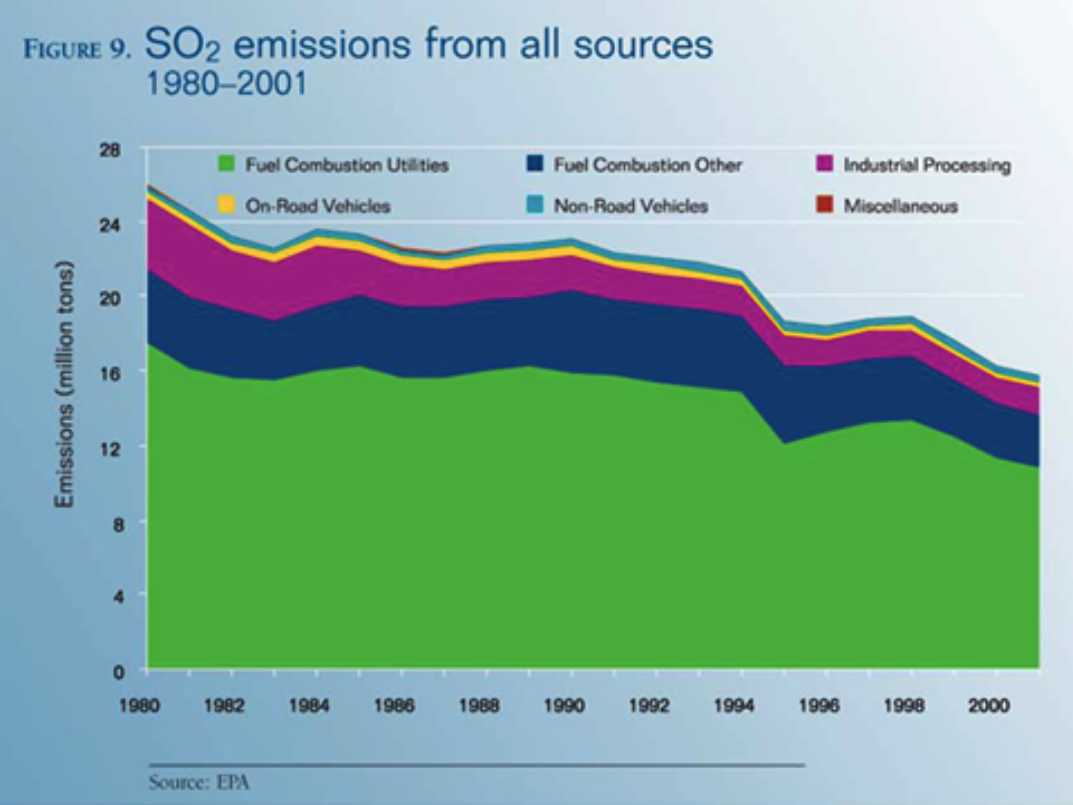
Source: National Science and Technology Council (18)
Figure 5: Trends in the Deposition of Acid Rain
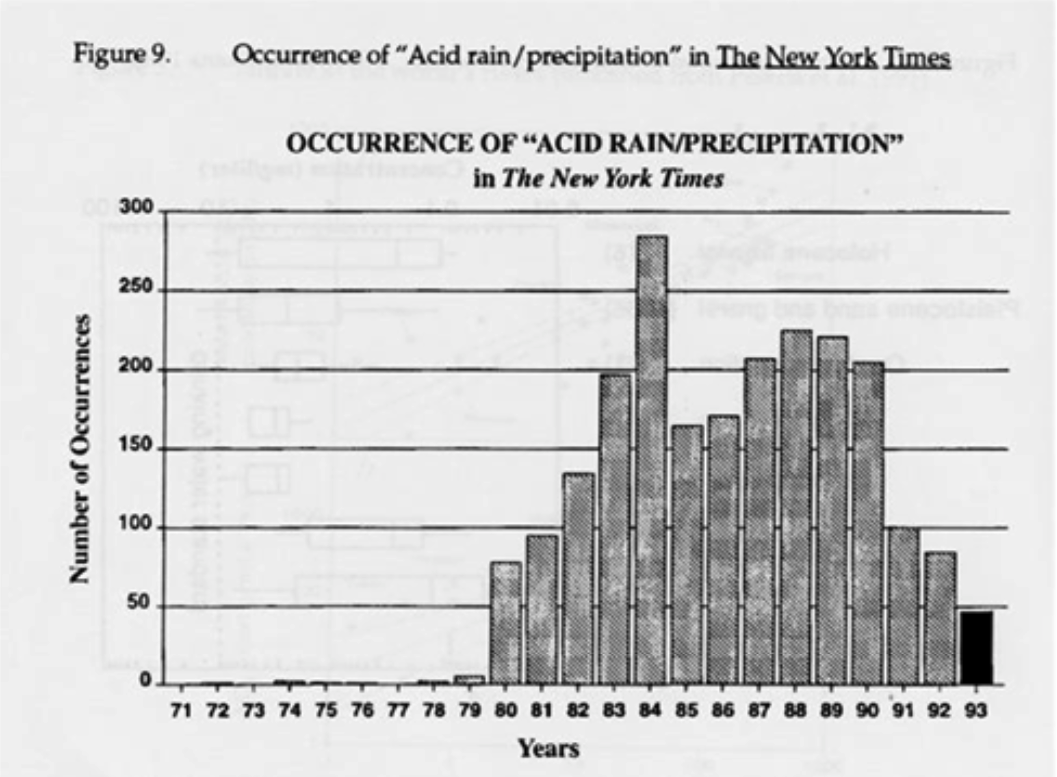
Source: Likens (19)
Hubbard Brook Experimental Station
The United States Department of Agriculture Forest Service established the Hubbard Brook Experimental Forest as a long-term research station on ecological studies. The research site is found in the White Mountains in New Hampshire.
Research interests in the station were composition and profile of the forest, disturbance reflex and aquatic ecosystems. Hubbard Brook gained prominence across North America as the first to experience acidic deposition.
Effects of acid deposition at Hubbard Brook reflect a forest ecosystem sensitive to acid inputs (Driscoll, Lambert and Chen 32). Over time, experimental activities and measurements regarding acid deposition and the aftermath on the ecosystem have been the dominant focal at the Hubbard Brook.
The lowering of sulfate concentration in rainfall has correlated with the rise in pH. Hubbard Brook has a long term inventory on precipitation chemistry. Over time precipitation records include bulk deposition date back to mid-1960s and wet deposition at the latter years of the 1970s (Driscoll, Lambert and Chen 32).
Conclusion made from the findings showed greater association between pollutant release levels of the precursor and the sulfur based acid deposition at the Hubbard Brook. It is thought that emission cuts at the source area would reflect linearly declines in sulfate deposition.
The eastern United States has provided a clear indication of the association between emission of precursor elements and the wet deposition. Over time in the period between 1984 -1986 to 2002-2004 high sulfate depositions has declined significantly in the eastern United States (Driscoll, Lambert and Chen 33).
The scenario of acid deposition reduction experience reflects the emission cuts targets entrenched in the 1990 CAAA.
Levels of nitrate or ammonium deposition have varied marginally at the forest station since 1963. Bulk deposition at down wind areas at Hubbard Brook has shown a direct association with the source area’s nitrogen oxide emission (Driscoll, Lambert and Chen 34).
Nevertheless, the association is feeble compared to sulfate. Inventories of nitrate emissions and the bulk deposition at the Hubbard Brook have had minimal change since experiments began in 1963.
The results of measurement generated at the Hubbard brook have reflected results obtained elsewhere in the eastern United States (Driscoll, Lambert and Chen 34).
Figure 6: Distribution Pattern for Sulfur Dioxide Emissions (in 1996)
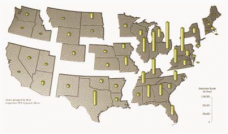
Source: The Adirondack Council (13)
Figure 7: Distribution Pattern for Nitrogen Oxide Emissions (in 1996)
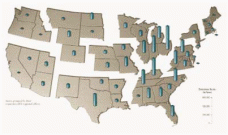
Source: The Adirondack Council (13)
Approaches to Acid Rain Abatement
The Act on Clean Air gave the preference to market approaches towards sulfur dioxide emission cuts. Industrial plants were supposed to adopt and obtain allowances from the emission cuts programs.
The market approach provides plants experiencing high emission scales opportunity to purchase sulfur dioxide credits from their counterparts whose emission costs are marginally lower. This approach has cost savings implications.
In contrast, use of the command-control approach proposed through environmental regulations may not amount to the same. Moreover, there are econometric functions within the market approach intended to measure the performance of sulfur dioxide allowance.
Assessing the level of cost (whether rising or falling) provides the indicator on performance emission cuts. On these bases, those plants that volunteer to use low-sulfur coal as an emissions-cut strategy, then the overhaul of the technology and decline in prices of sulfur containing coal have demonstrated the reduction in the marginal reduction of costs by more than half since 1985.
This forms the main bases for reducing cost other than trading. The strength of allowance approach is the cost savings of up to US$ 800 million annually. The command and control approach relies on public awareness and the establishing of an obligatory flat rate of emissions.
While it is imperative to consider the gains made by the market approach vis-a-vis the command-control; there is need not to lose sight of the overall necessity is not commercializing the whole affair but sustaining the health of the ambient atmosphere.
The doubling of the two approaches to emission cuts may lead more effective results other than taunting of one. Arguably, market approach may be the only acceptable within a particular jurisdictional area.
The fact that emissions and depositions drift from the source areas makes the sense that command control approach is more applicable for trans-boundary settlements on emission cuts.
Conclusion
There are categories provided for atmospheric compounds. Sulfur and nitrogen species are among the four categories. Emissions have led to cyclic atmospheric processes for nitrogen and sulfur pollutants. This has been described in the biogeochemical cycle.
The impact cyclic processes have traversed terrains and water masses. This means acid rain has a spatial and temporal perspective to it. Acid rain was first conceived by an English Chemist, but the concept earned popularity after twenty years.
This implies that the adverse impacts of acid deposition were not immediately perceivable thus the quality of the ambient environment is relative to placement and time. Acid rain has shown the ability to replicate the problem as well as traverse the space.
Nevertheless, this property of the acid rain and its precursors makes it difficult to particulaandr opt for one of the emission cut strategy (market or command-control approaches).
This is evident in the failure to reach at an amicable solution towards emission from the mid-eastern of North America source area and downwind in the Far East of the country. Based on the study argument most of the effort present alternative solutions towards reducing the amount of sulfur dioxide emitted to the air rather than absolute zero emissions.
The market approach considered as a preferable fails to campaign directly for zero emission of precursors but rather lower. Anecdotally, with the increasing establishment of more industrial plants the intensity of release may be low but the number sources increases translate to escalation of emission of precursors.
The role of the Hubbard Brook experimental outcomes is a clear indication that acid rain has a biogeochemical cycle. This puts the source and the downwind as important players towards providing solutions towards the acid rain phenomenon.
Actually, the drifting of acid deposition indicates that a porous solution towards an environmental problem can lead transferred to a second party. Providing longer smokestacks for releasing smoke implies that the environment at the troposphere is constantly mobile and that the atmosphere is constantly circulating and exchanging matter across the space.
In other words, the solutions towards the acid rain may not be transferring the emissions into the outer space but getting robust strategies to avoid the release of precursors at the source points. Solutions to the problem begin with the technologies applied in utilizing raw resources.
In addition, industry players should be prepared to embrace technology transfer for the common good. The market approach demonstrates that players in the same industry can participate in distributing and sharing an environmental problem resulting in significant reduction of emissions.
In the same vain technologies that prove workable towards lowering emissions can be shared as a way of corporate social responsibility, particularly in mitigating problems arising from emission release.
The market approach demonstrates that solutions to most environmental problems are best tackled through integrated approaches than independent players taking individual actions.
It is evident that corporate leaders have dominated in the technology front and have the capacity to institute emissions cuts with ease compared to small scale players. Thus, certain calls for emission cuts may not have an equal impact within the same industry.
Works Cited
The Adirondack Council 1998, Acid Rain: A Continuing National Tragedy. Web.
Downing, Robert, Ramesh Ramankutty and Jitendra Shah. RAINS-ASIA: An Assessment Model for Acid Deposition in Asia, Washington, D.C.: The World Bank, 1997. Web.
Driscoll, Charles, Kathy Lambert and Limin Chen. “Acidic Deposition: Sources and Ecological Effects.” Acid in the Environment: Lessons Learned and Future Prospects. Ed. G. Visgilio & D. Whitelaw. USA: Springer, 2007. 27-58. Web.
EIR/LaRouche Youth Movement Economics Team 2006, Retool Auto To Save U.S. Industrial Heartland. Web.
Likens, E. 1994, Human-Accelerated Environmental Change – An Ecologist’s View. Web.
Lind, Douglas 1981. Umbrella Equities: Use of the Federal Common Law of Nuisance to Catch the Fall of Acid Rain. PDF file. Web.
Mair, Andrew, Richardd Florida and Martin Kenney. “The New Geography of Automobiles Production: Japanese Transplants in North America.” Economic Geography. 64.4 (1988): 352-373. JSTOR. Web.
Mehta, Prashant. “Science behind Acid Rain: Analysis of Its Impacts and Advantages on Life and Heritage Structures.” South Asian Journal of Tourism and Heritage. 3 (2010): 123-132. South Asian Journal of Tourism and Heritage. Web.
National Science and Technology Council 2005, National Acid Precipitation Assessment Program Report to Congress: An Intergrated Assessment. Web.
Seinsfield, John and Spyros Pandis. Atmospheric Chemistry and Physics: From Air Pollution to Climate Change, USA: John Wiley & Sons, Inc. Web.
“The Industrial Heartland of North America”. Web.
Wang, Wenxing and Tao Wang. “On The Origin And The Trend Of Acid Precipitation In China.” Water, Air and Soil Pollution. 85 (1995): 2295-2300. Springer. Web.