- Chemical characteristics of the amino acid created
- The primary, secondary, tertiary and quaternary structures
- How a peptide bond is made through dehydration
- How a peptide bond is broken through hydrolysis
- Forces that stabilize a protein’s three-dimensional native structure
- The role of protein misfolding and aggregation in BSE
- The role of prions in BSE
- The role of the chaperone protein in BSE
- Recommendations on how to decrease the risk of BSE infecting the food sources
- References
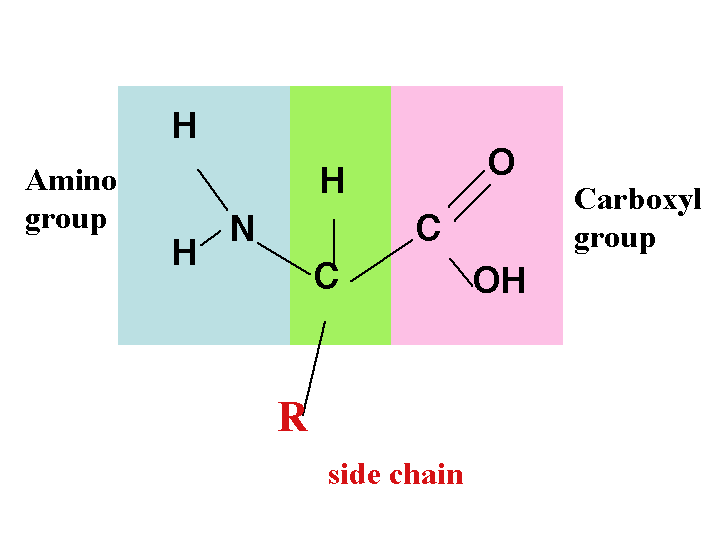
Chemical characteristics of the amino acid created
Every amino acid has a similar fundamental structure, which only differ in their side-chains. This side chain is usually designated the R-group. The side chain and the carboxyl group attach to the carbon atom, which is referred to the alpha carbon atom (Ca). In some cases, the R-group may only be a hydrogen atom. They may also be charged due to their dissociation or association of an amino group or a carboxyl group with a proton (Pauling, Corey, & Branson, 1951).
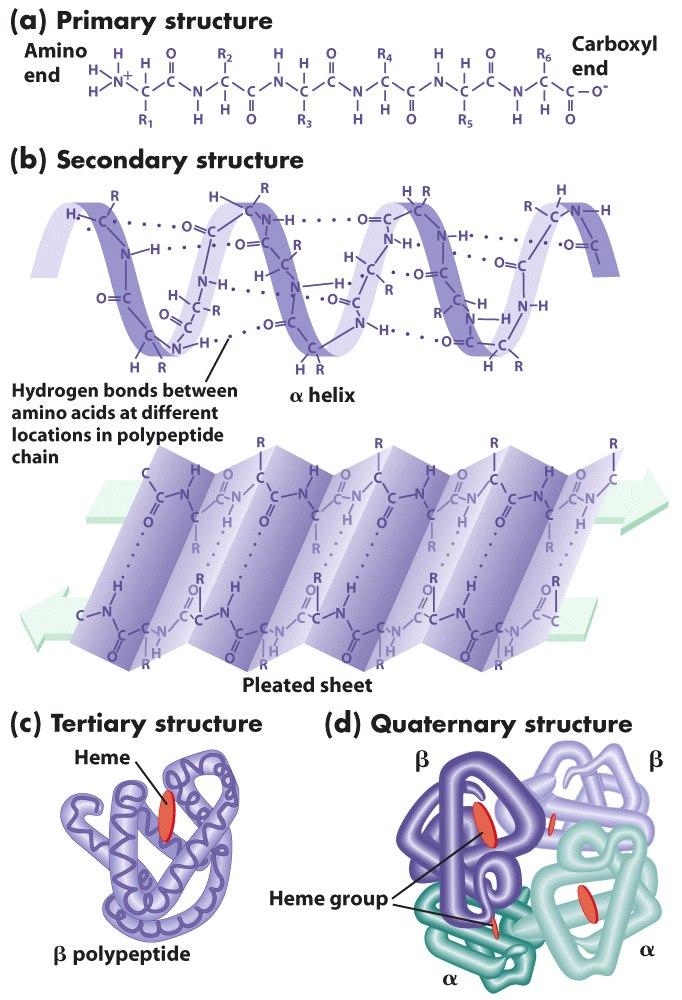
The primary, secondary, tertiary and quaternary structures
The primary structure of a protein is the sequence of covalently linked amino acids. These amino acids are linked within the proteins by the peptide bonds. The peptide bonds link the many amino acids to form the polypeptides (proteins). The protein secondary structure consists of two main types; the alpha helix and the beta strand and was suggested by Pauling, Corey and Branson (1951). This structure is defined by the patterns of hydrogen bonds. These bonds appear between the main chains of the peptide groups.
The tertiary structure is the three dimensional structure of a single molecule of a protein. The beta-sheets and alpha helices are usually folded to form a compact structure. This folding process is not driven by a specific hydrophobic interaction. The structure can only be stable if the parts of the protein domain are kept in place by the various tertiary interactions. They may include the salt bridges, the disulfide bonds or the hydrogen bonds. The quaternary structure is a large group of many polypeptides. The disulfide and non-covalent bonds mainly stabilize this structure.
How a peptide bond is made through dehydration
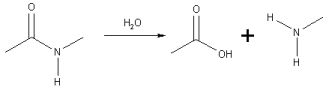
Peptide bonds are formed between two molecules as the carboxyl group of one of the molecules reacts with an amino group of the other. This causes the release of a molecule of water and therefore, this process is called a dehydration synthesis reaction. It can also be referred to as a condensation reaction. The formula of the resulting peptide bond is C(O)NH. The resulting molecule is called an amide. A peptide link is a functional group made up of four different atoms and is represented by the formula, -C(=O)NH-. Amino acids for chains called proteins, which are held by peptide bonds.
How a peptide bond is broken through hydrolysis
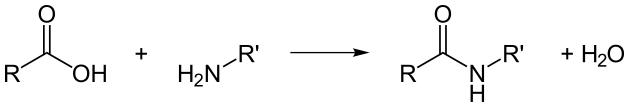
The breaking of a peptide bond is by the process of amide hydrolysis. Hydrolysis is the adding of water. In the presence of water, the peptide bonds break spontaneously to release energy. This property of breaking spontaneously in the presence of water is what leads to the naming of the peptide bonds as metastable. However, this process is a slow one. Enzymes might act as catalysts for this process when it occurs in living organisms (Martin, 1998).
Forces that stabilize a protein’s three-dimensional native structure
Four forces stabilize the three-dimensional structure of a protein. The first fore is the hydrogen bonding. Polypeptides have many proton acceptors and donors in their backbone and in the R-groups. The areas where proteins are located contain the Hydrogen bond acceptors and donors of the water molecule. Therefore, the hydrogen bond occurs between and within the chains of polypeptides and even around the aqueous medium. Other forces that stabilize the structure are the hydrophobic forces. The amino acids in the proteins may contain either a hydrophobic or a hydrophilic R-group. The interaction of the various R-groups in the aqueous surrounding plays an important role in the formation of the protein structure (Pauling, 1960).
Electrostatic forces also aid in the stabilization of the protein structure. They may be of three types; dipole-dipole, charge-dipole or charge-charge. The other forces that hold firm the structure of the protein are the van der Waals Forces, which are both attractive and repulsive and they control protein folding.
The role of protein misfolding and aggregation in BSE
The prions are the infectious particles, which are mainly composed of a single protein. They are the likely causative agents of many transmissible diseases such as the Bovine Spongiform Encephalopathy (BSE) that cause the deadly decline of the motor and cognitive functions. Research indicates that the protein responsible for this arrives at the pathogenic state through the process of misfolding from its original form (Anderson et al., 1996). Various characteristics of the process of aggregation require that the nucleus be formed prior to the stability of the conversion process. The slow process of nucleation would then allow the infected wound of an animal to introduce a step that initiates aggregation.
The role of prions in BSE
Prions are the likely causative agents of many transmissible diseases such as BSE. This disease is a transmissible spongiform encephalopathy (TSE). They are novel infectious particles and are usually single protein molecules. The prion is usually a misfolded protein and they carry the disease between organisms and cause deteriorative effects to the brain. The disease-causing shape for the misfolded protein occurs when the normal protein molecule contorts itself from the alpha helical arrangement to a beta-pleated sheet. This is caused when the animal carries an allele that is responsible for that process.
The role of the chaperone protein in BSE
The chaperone protein in BSE is responsible for the non-covalent unfolding or folding of various macromolecular structures. However, this does not occur when there is a normal biological function leading to the complete process of folding but it occurs when the protein folding is incorrect.
Recommendations on how to decrease the risk of BSE infecting the food sources
The discovery of the mad cow disease came when the new illness was noticed in many cattle with similar neurological symptoms. Later on, the disease was determined to be caused by the feeding of cattle with supplements made from sheep remains that were infected with such a disease. This means that the disease was transmitted from the sheep to the cows. The best way to avoid this is by not making the supplements from the remains of infected material.
Research has also determined a link between a human disease known as the Creutzfeldt-Jakob disease and the BSE from cattle. This disease is transmitted when one feeds on beef from infected cattle. The cow might have been infected with the BSE disease. I would recommend any country that has reported cases of the BSE disease within that country to discourage people from feeding on the meat form an infected animal. People should be made aware of the transmissibility of the disease and the dangers associated with it.
References
Anderson, R., et al. (1996). Transmission dynamics and epidemiology of BSE in British cattle. Nature, 382(1), 779-778.
Martin, R. (1998). Free energies and equilibria of peptide bond hydrolysis and formation. Biopolymers, 45(1), 351-353.
Pauling, L. (1960). The nature of the chemical bond. New York: Cornell University Press.
Pauling, L., Corey, R., & Branson, H. (1951). The structure of proteins; two hydrogen-bonded helical configurations of the polypeptide chain. Proceedings of the National Academy of Science, 37(4), 205-211.