The moisture content in food depicts its physicochemical properties, such as polarity, hydrogen bonding, boiling, melting points, and heat capacity. The removal of water in foods is associated with increased quality and as a preservative method. Moreover, food’s moisture content predicts how it should be stored, perishability, properties, and concentration of soluble components.
Aim
The experiment aims at analyzing moisture and its role in the storage and quality of foods.
Methods
Moisture or water content in food and its components can be measured using gravimetric, chemical, and spectroscopic approaches. The procedures were determined by the American Association of Cereal Chemists. Moisture content and water activity were measured at the different temperatures on jam, bread, meat, and fish food. The samples were first weighted on a muffin liner before dried in an oven for two hours at 135 degrees Celsius before being remeasured. Water activity shows the chemical properties of moisture in the food, ranging from 0.0 to 1.0.
Water content was also measured through weight comparison before and after drying. The food sample used had to attain a constant weight before taking the weight measurements. Such a process increased the validity of the moisture analysis. Moreover, moisture sorption isotherm was determined for wheat flour at 20 and 50 degrees Celsius by showing the relationship between moisture content and water activity.
Results
Fish food had the least moisture content (6.7%) and the lowest water activity of 0.530 at 25.1 degrees Celsius. The meat had the highest moisture content (61.1%) and a high level of water activity of 0.997 at 23.1 degrees Celsius, see Table 1.
Table 1. Moisture Content and Water Activity
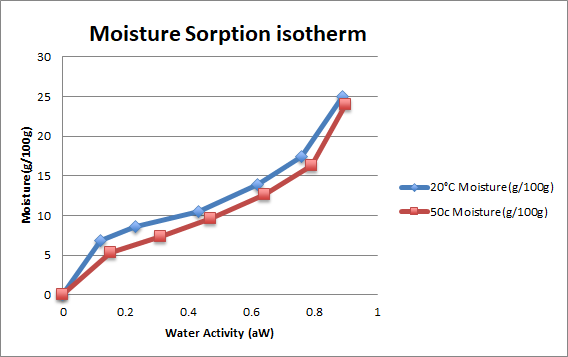
From the moisture sorption isotherm, the highest water activity was recorded at the highest moisture concentration, see Figure 1.
From the gaseous water experiment, soaked corn was observed to have the highest moisture content, and oven-dried corn had the lowest moisture content, illustrated in Table 2.
Table 2. Gaseous Water Experiment
Discussion
Moisture analysis is a critical measure in the food industry. The findings are as follows:
- The moisture content in food samples significantly impacted weight and texture, with dried food having the lowest weight, such as Meat had the highest, and Fish Food the least (See Table 1).
- The implications of moisture content go beyond weight to legal and labeling requirements, the shelf life, quality, and processing that are useful to consumers. Figure 1 shows water activity of wheat flour at different temperatures of storage.
- Deviations in moisture content have adversely effects on the quality of food products and volume. Corn with higher moisture content has poorer quality compared to that with lower levels of moisture (See Table 2).
- Water activity has been utilized as the best indicator for microbial growth in food samples. Food companies need to use a limiting water activity level to suppress such pathogenic bacteria’s growth. Freezing can be applied to reduce water activity by increasing volume (see Experiment 3).
- Water in food products increases the rate of microbial activity that causes spoilage or intoxication.
Conclusion
The moisture content analysis in food is vital to the food industry success since it determines the quality of products and their shelf life and compliance to legal requirements. Using the data on moisture content and water availability, food manufacturers can make vital decisions to ensure product safety and consistency in the market.
Reference List
Galanakis, C, Food Quality and Shelf Life, Academic Press, Cambridge, 2019.
Joardder, Mohammad U., Monjur Mourshed, and Mahadi H. Masud. State of Bound Water: Measurement and Significance in Food Processing. Basingstoke, Springer, 2018.
Nielsen, S. S. Moisture Content Determination. Food Analysis Laboratory Manual, 2017, 105-115.
Appendix 1. Formulas for Calculation
Moisture Content
% moisture = [mass of moisture lost (g)/ initial mass of food (g)] x 100
Water Availability
aw = P/Po = ERH (%) / 100
where:
- P = vapor pressure of water over a sample at a specified temperature
- Po = vapor pressure of pure water at the same temperature
- ERH = equilibrium relative humidity