Generally, liquids expand on heating and contracts on cooling. Water is a liquid which also expands on heating and contracts on cooling. However, when water is cooled, it begins to contract until the temperature reaches 4 degree Celsius, following which it begins to expand until it reaches 0 degrees. This expansion of water as it is cooled is not usual since most substances, especially liquids contract when they are cooled. Due to this abnormal expansion of water, the density of water is greater at 4°C than when it is at 0°C. It is for this reason that ice floats on water and freezing in ponds, lakes and other water bodies occurs foremost at the surface level gradually progressing downwards, which is also the crucial reason why aquatic life survives even at extremely low temperatures.
This paper aims to experiment and analyse this anomalous expansion of water which occurs when water is cooled from a temperature of four degree Celsius to zero degree Celsius.
Procedure/apparatus 5 NOT A COOKBOOK OR SET OF INSTRUCTIONS. Give a one paragraph description of how you went about testing your hypothesis
In order to test the hypothesis that water displays unusual characteristics in its expansion and contraction, the following procedure and apparatus were utilized. Water (100ml) was boiled to a temperature of about hundred degrees in a flask and as soon as it reached its boiling point, the heat was removed and the water in the flask was measured using a measuring jar. This water was allowed to cool and after it came to room temperature, all the water in the flask (100ml) was filled in a dish and its level was marked. The dish was then placed in the ice chamber of the refrigerator. The temperature of the water was regularly measured using a thermometer as the water began to cool. It was noted that as the water began to cool from about ten degree Celsius and became substantially cooler it began to contract. This was visible by noting the drop in level of water in the flask, which had been marked. However, the temperature of the water began to reduce and when the temperature dropped to 5 degree Celsius, the research noted the change in temperature every five minutes. It was found that when the water reached a temperature of four degree Celsius, it began to expand. This was noted by the increase in volume of the water in the flask, which showed an increase, well above the level which had been marked when the experiment was initiated. The volume of water in the flask had increased substantially, rather than reducing, which showed that water had expanded on cooling as opposed to the normal tendency of most substances to contract on cooling.
Interestingly, when this frozen form of water, ice, was again removed from the refrigerator and heated in the measuring beaker, it showed a reduction in volume. Thus, when the water had been heated above a temperature of four degree Celsius, it showed a decrease in volume from its volume between zero and four degree Celsius.

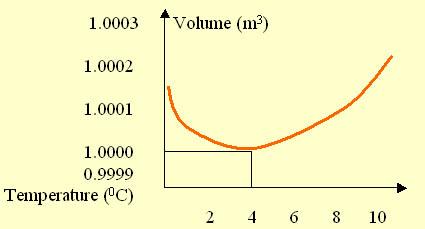
Raw Data 5 Give your experimental results in the form of TABLES or GRAPHS (CHARTS). AND Describe the information in your tables & graphs.
The above graph is a clear representation of the unusual expansion of water with changes in temperature. The volume of water relative to the changes in temperature is depicted above in the graph. The curve which is formed denotes the reduction in volume of water as the temperature increases from zero degrees to about four degrees Celsius. After four degree Celsius, the curve changes its direction and moves the other way round which indicates that the volume of the water increases wit temperature once again. The experiment and the data prove that water expands anomalously when it is cooled from four degrees to zero degrees, through the increase in volume which occurs.
The data and findings support the hypothesis that between zero and four degrees, water depicts unusual properties and expands, rather than contracting, and increases in volume. The measure of water was observed during the entire process and the sudden change in the volume of water was noted. This investigation proves the hypothesis that water expands anomalously when cooled and increases in volume as it nears its freezing point of zero degree Celsius.