Atherosclerosis
Atherosclerosis is a process of progressive hardening of the arterial wall and elasticity loss. It is a slow process that begins in childhood and needs variable time to manifest itself as a health problem. Usually, it affects large- and medium-sized arteries. It is characterized by atheromatous deposits, thickening, and fibrosis of the inner layers of the arterial wall; forming what is known as atherosclerotic plaque. It can be investigated by direct methods such as angiography, by recognizing the risk factors responsible for developing it, or by recognizing the complications resulting from it.
Incidence and Prevalence
Atherosclerosis is considered a leading cause of vascular morbidity worldwide. It is complicated by several serious conditions such as ischemic heart disease, ischemic stroke, and peripheral arterial disease. However, some high-income countries have reported a decline in the incidence and mortality from such serious conditions, which could be explained by the recognition of many risk factors for atherosclerosis and modifying the lines of treatment. On the other hand, most developing countries of low- and middle-income have reported a decline in mortality from ischemic stroke, but variable mortality rates from ischemic heart disease (Babu, Nagaraja, & Reddy, 2016; Herrington, Lacey, Sherliker, Armitage, & Lewington, 2016). The prevalence rate of atherosclerosis increases with age. Males are affected more than females, but they are almost equal in the age group of 60 years and above. Also, significant lesions are observed in chronic smokers, alcoholics, and non-vegetarians.
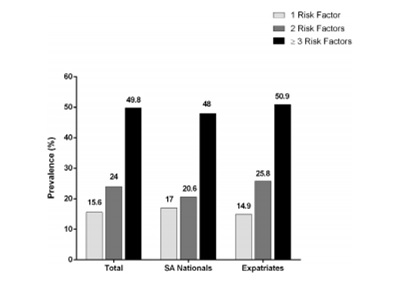
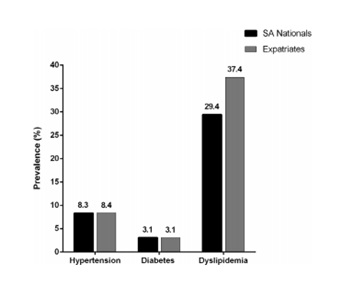
In Saudi Arabia, the major economic transition and rapid urbanization witnessed a significant rise in the burden of cardiovascular diseases. Many studies revealed a high prevalence of obesity and dyslipidemia, which are considered major risk factors for developing atherosclerosis, among the population. A cross-sectional study was conducted to evaluate the prevalence of important cardiovascular risk factors among adult Saudi patients attending primary care clinics. Comparisons were made between different groups of the studied sample. The study showed that dyslipidemia was the most prevailing risk factor (Ahmed et al., 2017). Figure 1 represents the prevalence of cardiovascular risk factors for every patient. Figure 2 represents the prevalence of the newly diagnosed cases of hypertension, diabetes mellitus, and dyslipidemia that were discovered during screening. “SA Nationals” is referring to patients of Saudi ethnicity, and “Expatriates” is referring to patients who were not of Saudi ethnicity.
In a cross-sectional study conducted on the students of Riyadh College of Health Science – male section, anthropometric measurements were taken, and some laboratory investigations were performed to assess the relationship between lipid profile and anthropometric measurements. The results showed a high prevalence of obesity; the mean value of body mass index (BMI) was 25.26 ± 5.86 kg/m2. BMI is widely accepted as an indicator of total adiposity, but it has some limitations that depend on race and age (Al-Ajlan, 2011; Al-Shehri, 2014). BMI of (≥25) indicates overweight, and of (≥30) indicates obesity.
The study also revealed positive correlation between BMI and total cholesterol (TC) and low-density lipoproteins (LDL), negative correlation between BMI and high-density lipoproteins (HDL), and no correlation between BMI and triglycerides (TG). Another cross-sectional study was conducted to investigate the prevalence and patterns of dyslipidemia among Saudi patients who did a coronary angiography and were documented to have coronary artery disease (CAD). The study revealed that 75% of patients with coronary atherosclerosis had dyslipidemia (Al-Shehri, 2014). There was a strong association between LDL levels and the severity of presentation of coronary artery disease. Also, low HDL levels represented the most frequent lipid abnormality, with significant influence on the extent of the disease.
Pathophysiology
Atherosclerosis is a chronic disease affecting arteries. A better understanding of the pathophysiology of atherosclerosis has changed its concept from a lipid storage disease into a chronic inflammatory disease. Atheroma formation was thought to be a passive lipid collection in the arterial wall, based on the presence of fatty streaks and lipid-laden cells (foam cells) in the arterial wall (Libby, 2012). However, the modern era of atherosclerosis has focused on the inflammatory process and the proliferation of smooth muscle cells in the arterial intima as a nidus for forming atherosclerotic plaques. These smooth muscle cells acquire the ability to migrate from media to intima in the arterial wall, proliferate, and change from the quiescent contractile state to the active synthetic state to share in extra-cellular matrix (ECM) accumulation.
Atherosclerosis as an Inflammatory Process
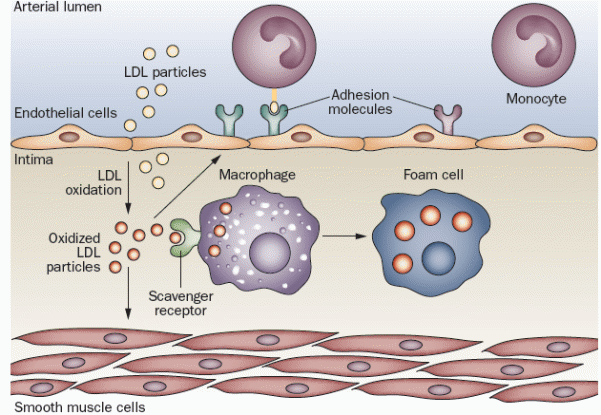
Inflammation has shown to be involved in all aspects of atheromatous plaque formation including inception, progression, and complication. Inflammation is defined as a local response to cellular injury. This response is characterized by capillary dilatation and inflammatory cells infiltration. Normally, the endothelial cells lining the arterial wall provide a dynamic interface between the bloodstream and the arterial wall, and provide a unique surface that allows laminar blood flow, resists adhesion of leucocytes and promotes fibrinolysis. Once exposed to damage, the inflammatory reaction is triggered based on the response to injury theory. The injured endothelial cells express adhesion molecules that recruit many classes of leucocytes (most importantly, blood monocytes). As blood monocytes adhere to the endothelium, they receive chemoattractant signals from pro-inflammatory mediators known as chemokines.
These signals drive them to enter the intima. Inside the intima, they mature into macrophages that express scavenger receptors. Scavenger receptors allow the macrophages to engulf modified lipoprotein particles, so they acquire the classic microscopic appearance of foam cells that appear in atherosclerotic lesions. Macrophages proliferate inside the intima and release many growth factors and cytokines, and then undergo apoptosis. Nitric oxide (NO) is produced by endothelial cells and macrophages as well. Depending on its source, it may act as an atherogenic or protective factor. Nitric oxide produced by endothelial NO synthase (eNOS) is vasodilator and protective, while NO produced by inducible NO synthase (iNOS) is atherogenic with potent oxidative properties; as it has antimicrobial functions (Libby, Okamoto, Rocha, & Folco, 2010). Figure 3 illustrates the process of inflammatory response and formation of foam cells. The injury to the arterial endothelium is accompanied by platelet adhesion, aggregation, and formation of a platelet plug.
These events also play a role in the formation of atherosclerosis, as platelets tend to release some inflammatory mediators that enhance the recruitment of the inflammatory cells to the site of the lesion. These changes lead to vascular modifications in the form of vessel narrowing and plaque instability that may eventually rupture (Lievens & von Hundelshausen, 2011). An analysis of these subsequent events suggests that the evolution of advanced plaques witnesses repetitive cycles of micro-hemorrhage and thrombosis. Plaque rupture results in exposure of plaque lipids and tissue factors to blood components, starting the coagulation cascade, platelet adherence, and thrombosis. Moreover, atherosclerotic plaques weaken the underlying media and may lead to aneurysm formation; adding to the possible serious cardiovascular complications.
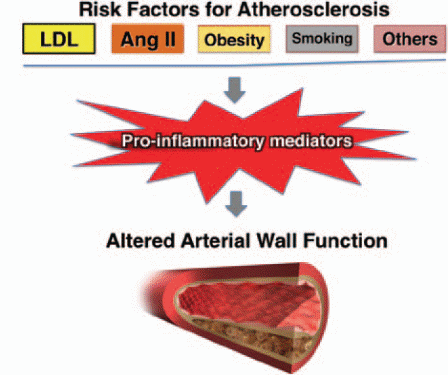
Prolonged exposure to cardiovascular risk factors leads to an imbalance between endothelial injury and repair. Injury to the endothelium means that the cells become dysfunctional, lose integrity, progress to senescence, and detach into the circulation. The circulating endothelial micro-particles can be used as markers of endothelial cell damage. The endothelial injury can result from various risk factors such as hypertension, dyslipidemia, obesity, insulin resistance, metabolic syndrome and cigarette smoking. Smoking is recognized to cause dyslipidemia, enhanced thrombosis, and increased platelet consumption in atheromatous lesions with a subsequent increase in mean platelet volume (MPV). In turn, this increase promotes atherosclerosis. These risk factors are considered the traditional risk factors for atherosclerosis.
Also, some hereditary and genetic disorders represent a cardiovascular risk factor; with early presentation of clinical manifestations of atherosclerosis. Despite considering atherosclerosis as an inflammatory condition, the link between these risk factors is still being recognized. In fact, they all share many common aspects of their pathophysiological concepts. Many studies have proven similarities between atherosclerosis and obesity-related metabolic disorders in their pathophysiology regarding the role of macrophages and inflammatory mediators and pathways. Also, various studies stated that plasma lipoproteins contain products known as lipid oxidation products (LOP), which alter the normal physiological functions and enhance the atherosclerotic process (Rocha & Libby, 2009). As a result, a new relationship between these risk factors and atherosclerosis has been established as shown in Figure 4.
The Role of Inflammatory Cytokines
An early step of inflammation is the release of chemokines such as monocyte chemoattractant protein (MCP)-1, also known as chemokine ligand (CCL2) that interact with chemokine receptor CCR2, and other factors. These factors increase the expression of adhesion molecules such as intercellular adhesion molecule (ICAM)-1, which has a role of allowing monocytes/macrophages to enter the arterial intima by diapedesis. MCP-1 is believed to have a very important role in atherosclerosis formation. It enhances the migration of circulating monocytes to the sites of inflammation and formation of atheromatous plaques. It is mainly concerned with monocytes chemoattraction, not neutrophils that have another chemoattractant known as interleukin-8 (IL-8) (Tabet et al., 2014).
It is considered a marker of adverse cardiovascular events, as its circulating level is greatly increased in patients with coronary or peripheral artery disease. It reflects the degree of endothelial dysfunction and atherosclerotic burden. Also, it is used as a marker for coronary inflammation because it mediates the atherogenic effects of other risk factors for coronary artery atherosclerosis (Rabkin, Langer, Ur, Calciu, & Leiter, 2013). Some experimental studies have stated that the deletion of MCP-1 prevents macrophage recruitment and atherosclerotic lesion development (Ma, Yabluchanskiy, Hall, & Lindsey, 2014). Furthermore, some studies have claimed that decreased expression of ICAM-1 is an important mechanism in the beneficial effect of certain agents on cardiovascular function such as resveratrol, which is a known anti-aging compound used in prevention and treatment of some chronic conditions (Agarwal et al., 2013). Another study stated that high-density lipoproteins (HDL) transferred microRNA-223 have induced a reduction in the endothelial activation by directly targeting ICAM-1.
Allograft Vasculopathy
Allograft vasculopathy represents another evidence for ending the old theory of considering atherosclerosis as a lipid storage disease, and evidence for the role of inflammation even in the absence of traditional risk factors (Mitchell, 2013). It is represented by an accelerated intimal hyperplastic lesion that leads to progressive vascular stenosis, so it is considered a major long-term limitation to successful transplantation of a solid organ. It involves donor arteries and spares host arteries. Hence, it is regarded as a special example of immune-mediated atherosclerosis.
Prevention
Apart from the non-modifiable risk factors such as age, genetic causes, and family history, other risk factors for atherosclerosis can be controlled to prevent or delay the occurrence of its clinical sequel. The mainstays for prevention depend on controlling the traditional risk factors and lifestyle modification. Healthy eating habits can improve the lipid profile and delay the progression of the disease. A healthy diet should be low in saturated fat, salt, added sugar and refined grains. It should be rich in fruits, vegetables, and whole grains. Regular aerobic exercise can reduce cholesterol level, control the body weight, and improve the fitness level. The type and amount of activity should be individualized according to the general condition of the patient. Quitting smoking is a very important factor in the prevention of atherosclerosis. Also, avoidance of passive smoking is advised (Babu et al., 2016). Smoking is known to promote atherosclerosis, and it is riskier if associated with other risk factors such as hypertension. Many programs and products are available to help quit smoking. Weight loss if needed helps improve the lipid profile and control other risk factors such as diabetes mellitus and hypertension. Moreover, appropriate control of diabetes and hypertension is an essential factor for delaying the clinical sequel of atherosclerosis.
Treatment
Many studies have proved that inflammatory mediators and cytokines, generation of reactive oxygen species (ROS) through activation of NADPH oxidase in a process known as oxidative stress, and the renin-angiotensin system (RAS) play a critical role in the pathophysiology of atherosclerosis. These new trends have paid attention to new treatment strategies such as anti-inflammatory agents, RAS blockers, and anti-oxidants in the treatment of atherosclerosis. Also, many new classes of drugs are still under research such as LDL-cholesterol (LDL-C) lowering therapy and other lipoprotein modification agents. The goal of treating atherosclerosis is not reversing the condition, but preventing it from getting worse. Once atherosclerosis is diagnosed, all preventive lines should be followed to protect against the serious complications (Husain, Hernandez, Ansari, & Ferder, 2015).
Moreover, statins (HMG-CoA reductase inhibitors) are still widely used, and many studies approve their efficacy. They are classified as lipid-lowering drugs; acting on LDL-C levels in the blood. They are beneficial in treating early stages of cardiovascular disease; working as secondary preventive agents. Also, they are beneficial in protecting susceptible patients with high levels of risk factors; working as primary preventive agents (Rabkin et al., 2013). Other drugs may be recommended in treating atherosclerosis depending on the associated risk factors. These drugs may include fibrates, anti-platelet medications, beta-blocker medications, calcium-channel blockers and anti-diabetic medications. Surgical procedures are not recommended in treating atherosclerosis unless complications occurred.
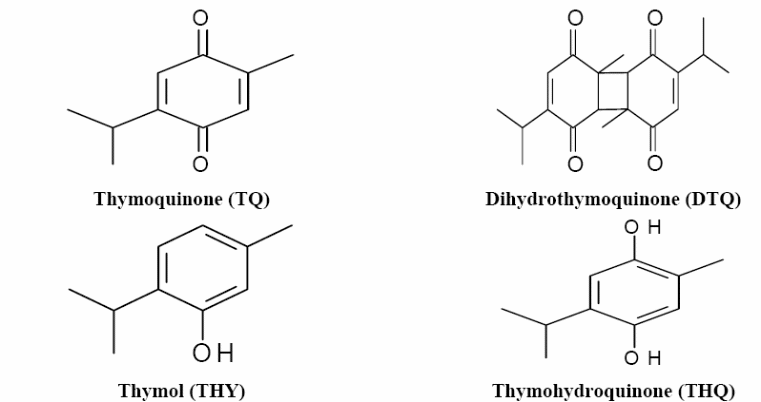
Thymoquinone
Thymoquinone is a phytochemical compound produced by Nigella Sativa. Its main function is to help plants thrive or impede pathogens, predators, or competitors. It has been used as a traditional medicine for many years (Glaser & Holzgrabe, 2016). Additionally, thymoquinone has been classified as one of the pan-assay interference compounds (PAINS). These compounds bind to different proteins and do not have a specific mode of action.
Source
Thymoquinone (TQ) is a derivative of a medicinal plant called Nigella Sativa (also known as black cumin or black seed). Nigella Sativa is an annual herb, native to South West Asia and cultivated in North Africa and Europe. Thymoquinone has the chemical formula of 2-Isopropyl-5-methyl-1, 4-benzoquinone. It has a historical and religious background. In the Islamic tradition, it has been used for its healing powers. Also, it has been used as a traditional medicine in the Middle and the Far East for treating several diseases such as bronchial asthma, headache, hypertension, dysentery, and gastrointestinal problems. Nigella Sativa contains many ingredients. It contains about 36% fixed oil, and about 0.45-2.5% volatile oil (Ragheb et al., 2009). Figure 5 illustrates the important active ingredients obtained from Nigella Sativa volatile oil by High-Performance Liquid Chromatography (HPLC) analysis. Thymoquinone is considered the major bioactive component among these active ingredients, as it has been subjected to many studies investigating its variable actions since its first extraction in the 1960s.
Metabolism
In vitro, Nigella differs according to the nature of the media. In neutral aprotic media, it produces the reversible semiquinone and the quasi-reversible quinone dianion. In an acidic pH, the ultimate product is the stable hydroquinone. In an alkaline pH, it produces quinone dianion (Islam et al., 2016). Regarding the pharmacokinetics of thymoquinone, a study was conducted to compare between oral (PO) and intravenous (IV) bioavailability of thymoquinone using a rabbit model. Thymoquinone showed a good tolerance in both routes of administration up to the targeted dose. PO administration showed a rapid elimination and a relatively slower absorption of the thymoquinone compound. This can be referred to a (flip-flop) model, which means that absorption T1/2 is longer than that of elimination. Also, the elimination phase reflects the input absorption rate constant (Ka), more than the output (Kel), which means that no elimination until absorption occurs; making Ka the rate-limiting step.
So, this causes an increase in thymoquinone half-life and suggests that PO administration can be proposed. Also, it showed a quick elimination after IV administration with a terminal apparent elimination half-life (T1/2λ) of ~1.0 h (Alkharfy, Ahmad, Khan, & Al-Shagha, 2015). The concentration-time profile of IV dose represented a bi-phasic decline in thymoquinone. This could be explained that the initial rapid decline resulted from rapid drug distribution phase with consequent binding to both plasma and tissue. Also, it showed a relatively small apparent volume of distribution at steady state (Vss), which might be due to high binding to plasma proteins and poor water solubility. Furthermore, thymoquinone-loaded nanostructured lipid carriers (NLCs) are investigated to be used in PO administration. NLCs are colloidal drug carriers, composed of solid and liquid lipids, and surfactants. They represent a promising vehicle for oral administration of thymoquinone, as they are assumed to improve the gastroprotective properties of thymoquinone.
Anti-Inflammatory Activity
During studying the airway inflammation in mouse and rat models of allergic asthma, thymoquinone showed an effective anti-inflammatory activity. It caused a reduction in prostaglandin D2 production in the airways, and a reduction in lung eosinophilia (Gholamnezhad, Keyhanmanesh, & Boskabady, 2015). Moreover, it decreased the production of T helper type 2 (Th2) cytokines in the bronchoalveolar lavage fluid and decreased cyclooxygenases (COX-2) protein expression in the lung. Also, it lowered the raised serum levels of IgE and IgG1 and suppressed the allergen-induced lung eosinophilia. Another experimental procedure was the induction of arthritis in rats. Administration of thymoquinone caused a marked reduction in both clinical and radiological arthritis scores, and a decline in tumor necrosis factor (TNF-α) and interleukin (IL-1β) as well. Administration of thymoquinone to female rats with induced gestational diabetes has resulted in improvement of diabetic complications in their offspring (de Cássia da Silveira e Sá, Andrade, & de Sousa, 2013). It produced an increase in the IL-2 level and T cell proliferation, improving the T cell immune response.
Another study investigated the anti-inflammatory effect of thymoquinone in vitro and in vivo as well. The in vitro study was conducted using lipopolysaccharide (LPS)-activated murine macrophage-like RAW264.7 and human monocyte-like U937 cells. The in viva study was conducted using LPS/D-galactosamine (GalN)-induced acute hepatitis, and hydrochloric acid/ethanol (HCl/EtOH)-induced gastritis in mice (Hossen et al., 2017). The study concluded that the anti-inflammatory effect is mediated through the inhibition of interleukin-1 receptor-associated kinase 1 (IRAK1)-linked activator protein (AP) −1, and nuclear factor (NF)-κB pathways.
Anti-Oxidant Activity
Reactive oxygen species such as superoxide anion radical (O2-), hydroxyl radical (OH-), hydrogen peroxide (H2O2), hypochlorous acid (HOC1-), and peroxynitrite (ONOO) are normal products of metabolic processes produced by NADPH oxidase enzymes. The high level of these ROS causes tissue damage. The anti-oxidant activity of thymoquinone is summarized in its capability of improving the action of anti-oxidant enzymes such as glutathione peroxidase, glutathione-S-transferase, and catalase enzymes, and acting as a free radical and superoxide radical scavenger. In a rat model of acute bacterial prostatitis induced by Escherichia coli (E. coli), thymoquinone exhibited a protective anti-oxidant effect against tissue injury (Gholamnezhad et al., 2015; Inci et al., 2013).
It produced a reduction in malondialdehyde (MDA) levels, which is regarded as one of the lipid peroxidation markers, and an improvement in the histological damage generated by E.coli. In rats with induced hypercholesterolemia, thymoquinone showed significant activity in scavenging hydroxyl radicals (OH-) from plasma. Also, it decreased Plasma total cholesterol and low-density-lipoprotein (LDL) levels. Also, it resulted in an up-regulation of catalase, superoxide dismutase 1 (SOD1) and glutathione peroxidase-2 genes, and an increase in the liver antioxidant enzyme levels (Amin & Hosseinzadeh, 2016; Harzallah et al., 2012; Ismail, Al-Naqeep, & Chan, 2010). Thymoquinone protected the erythrocytes from lipid peroxidation in rats with 1, 2-dimethylhydrazine- (DMH-) induced colon carcinogenesis.
Anti-Atherogenic Activity
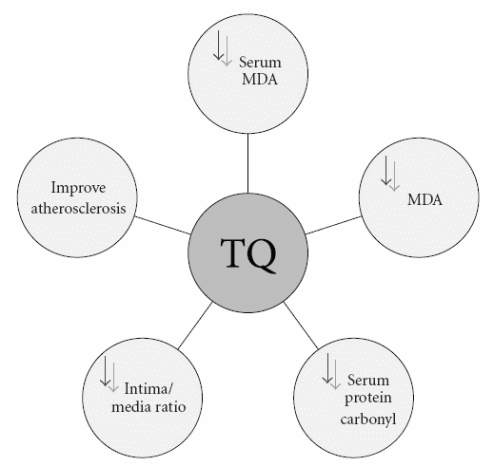
The anti-atherogenic effect of thymoquinone emerges from the fact that it has anti-oxidant properties and it can improve the lipid profile. The hypolipidemic effect of thymoquinone is due to the inhibition of de novo cholesterol synthesis, and the stimulation of bile acid excretion (Farkhondeh, Samarghandian, & Borji, 2017). It causes a decrease in the high levels of LDL, total cholesterol, and triglycerides (TG), and causes an increase in high-density lipoproteins (HDL) level. Therefore, it promotes ameliorating oxidative stress-induced atherosclerosis (Farkhondeh et al., 2017). In a rabbit model of atherosclerosis, atherosclerosis was induced by hyperlipidemia and aggravated by administration of cyclosporine A (CsA). Thymoquinone exhibited anti-atherogenic properties and inhibited the formation of atherosclerotic plaque by 50% (Farkhondeh et al., 2017).
This significant result was investigated by many parameters. One of the interesting parameters was studying the intima/media ratio, as thymoquinone caused about 75% reductions in the intimal cross-sectional area and the intima/media ratio (Ragheb, Attia, Elbarbry, Prasad, & Shoker, 2011). Figure 6 shows the different mechanisms of action of anti-atherogenic activity of thymoquinone. Nigella Sativa oil was found to regulate the cell growth and differentiation of monocyte and monocyte-derived macrophages when isolating primary human monocytes from whole blood. It reduces the production of pro-inflammatory mediators by monocyte-derived macrophages (Shabana, El-Menyar, Asim, Al-Azzeh, & Al-Thani, 2013). Also, thymoquinone causes some improvement in the endothelial dysfunction via anti-oxidant effect and normalization of the angiotensin system. In addition, it was proved that Nigella Sativa seeds inhibited the arachidonic acid-induced platelet aggregation.
Anti-Cancer Activity
Thymoquinone is thought to attain its anti-cancer properties through different modes of action. Several studies have investigated these different mechanisms which include anti-proliferation, cell cycle arrest, apoptosis induction, anti-angiogenesis, anti-metastasis, generation of ROS, oxidative damage of cellular macromolecules, inhibition of carcinogen-metabolizing enzyme activity, activation of caspases, and modulation of many molecular targets as peroxisome proliferator-activated receptors gamma (PPAR-γ) and signal transducer and activator of transcription 3 (STAT3) (Woo, Kumar, Sethi, & Tan, 2012).
A study on survival and apoptotic pathways in cell lines of acute myelogenous leukemia (AML) has revealed that thymoquinone produced anti-proliferative and pro-apoptotic effects on AML cell lines, and caused an increase in p53, and caspase-3 and -9 expression levels. Moreover, pre-exposure to thymoquinone caused an increase in cell growth inhibition (Khalife, Stephany, Tarras, Hodroj, & Rizk, 2014). Furthermore, thymoquinone has proved efficacy in another type of leukemia; adult T-cell leukemia. Adult T-cell leukemia (ATL) is caused by human T-cell lymphotropic virus 1 (HTLV-1). Doxorubicin is a chemotherapy that has serious side effects such as developing drug resistance and cardiotoxicity (Gali-Muhtasib, Fakhoury, Fatfat, Mismar, & Schneider-Stock, 2017). The combination of thymoquinone and doxorubicin proved to cause ALT cell line death by apoptosis. This effect was at lower doses of doxorubicin, which offers an opportunity to reduce its serious side effects.
Another mechanism of action is anti-metastasis. Cancer metastasis is associated with epithelial to mesenchymal transition (EMT), and it is believed to be the leading cause of death in cancer patients. Thymoquinone has been suggested to inhibit bone metastasis of breast cancer in a mouse model. The same suggestion is applied for the metastasis of melanoma cells. A study conducted on cancer cell lines proved that thymoquinone inhibited the expression of EMT mediator proteins and their regulatory transcription factors (Khan et al., 2015). Colorectal cancer is considered one of the most common cancers in developed countries. Thymoquinone was reported to reduce the viability of human colon cancer HCT116 cells, and this significant effect was in a concentration- and time-dependent manner. One of the hallmarks of cancer is the ability of cancer cells to bypass apoptosis; thymoquinone exerted its anti-cancer effect via induction of apoptosis (Kundu, Choi, Jeong, Kundu, & Chun, 2014). The induction of apoptosis is mediated through blocking STAT3 signaling through the inhibition of Janus-activated kinase-2 (JAK2) and Src kinase-mediated phosphorylation of epidermal growth factor receptor (EGFR) tyrosine kinase.
The introduction of nanoparticle approach is under research to enhance the effectiveness and bioavailability of thymoquinone. Thymoquinone nanoparticle (TQ-NP) has shown more effectiveness than thymoquinone regarding anti-proliferative, anti-inflammatory, and chemo-sensitizing properties. It shows more potency in making leukemic cells more sensitive to tumor necrosis factor (TNF) and in inhibiting the proliferation of many cancer cell types such as colon, prostate, breast cancer, and multiple myeloma cells. It shows more activity in inhibiting the expression of matrix metalloproteinase (MMP)-9, markers of cell proliferation, and vascular endothelial growth factor (VEGF) (Ravindran et al., 2010). Many researchers studied the anti-cancer activity of thymoquinone and suggested that combination of thymoquinone with other chemotherapeutic agents resulted in more potent effect, and decreased the undesirable side effects of chemotherapeutic agents; as they can be used in lower doses.
Other Actions of Thymoquinone
Many studies investigated the anti-microbial activity of thymoquinone against several bacterial, viral, and parasitic organisms. For example, it is suggested that it can inhibit Streptococcus mutans and other oral bacteria, protecting against dental caries (Chaieb, Kouidhi, Jrah, Mahdouani, & Bakhrouf, 2011). Also, more studies are being conducted to prove its ability to be a source of natural products with resistance-modifying activity. In a study conducted to investigate the antibacterial activity of thymoquinone, it showed a significant bactericidal activity against a wide range of tested bacteria especially gram-positive cocci (Chaieb et al., 2011). This result supported previous results of experimental studies, which also stated that combination of thymoquinone with antibiotics exerted a synergistic effect. Thymoquinone prevented the biofilm formation, which is a group of cells attached to the biotic or abiotic surface, providing hostile environmental conditions for survival of micro-organisms, and the released pathogenic bacteria lead to food hygiene problems. Thymoquinone mediated this effect through anti-oxidant activity.
Thymoquinone has been supposed to possess a hepatoprotective effect. Thymoquinone exerted a hepatoprotective effect against tert-butyl hydroperoxide (TBHP) toxicity by preventing depletion of GSH and protecting the liver enzymes from leakage. Also, it protected against tamoxifen-induced hepatotoxicity by normalizing the activity of superoxide dismutase, preventing the rise in TNF-α, and decreasing the histopathological changes. It protected against Lead (Pb) induced hepatic toxicity. In addition, it is supposed to protect against carbon tetrachloride (CCl4) induced hepatic toxicity, and to act as an anti-fibrotic agent by directly affecting hepatic stellate cells (HSCs) and down-regulating the mRNA expression of many fibrosis-related genes (Nagi, Almakki, Sayed-Ahmed, & Al-Bekairi, 2010). Thymoquinone is reported to exert a hepato-renal protecting effect on methotrexate toxicity. Another study aimed to assess the ability of thymoquinone to guard against acetaminophen-induced hepatotoxicity in mice. Pretreatment with thymoquinone resulted in a dramatic decrease in serum alanine aminotransferase (ALT) activities in a dose-dependent manner. This effect is explained by anti-oxidative and anti-nitrosative activity, and by enhancing the mitochondrial energy production.
Anti-diabetic effect of thymoquinone was extensively investigated in many studies. The hypoglycemic effect of thymoquinone was supposed to result from the anti-oxidant properties, which lead to the preservation of pancreatic islet cells and glucose hemostasis. Other proposed mechanisms include decreasing gluconeogenesis and inhibition of intestinal absorption of glucose. Also, it might have insulin-like effects at the cellular and molecular levels in different organs such as skeletal muscles. Available information suggests that macrophage inflammatory products usually increase in acute type I DM, and decrease in chronic type II DM. Thymoquinone is reported to reduce the raised levels of these inflammatory mediators to the normal levels (Abdel-Zaher et al., 2013; Islam et al., 2016). Thymoquinone can play a role in attenuating the development of morphine tolerance and dependence.
On studying morphine-induced tolerance and dependence in mice, thymoquinone inhibited the naloxone-induced rise in brain MDA and NO levels, but it did not affect the naloxone-induced rise in brain glutamate. Thymoquinone is supposed to have a protective effect against nephrotoxicity induced by drugs. Some studies stated that supplementation of thymoquinone in drinking water decreases susceptibility to drug-induced renal abnormalities. For instance, it was reported to protect against gentamicin (GM) induced nephrotoxicity. Cyclophosphamide (CYP) is reported to induce hemorrhagic cystitis, which is a dose-limiting side effect. Thymoquinone was proved to ameliorate the CYP-induced hemorrhagic cystitis in an experimental study using mice (Gore et al., 2016). This protective effect resulted from the ability of thymoquinone to decrease the oxidative stress reactions, inhibit the fragmentation and damage of the DNA, and increase the expression of a nuclear factor related erythroid 2-related factor (Nrf2) in the bladder tissues.
Thymoquinone exhibited dose‑dependent apoptosis in head and neck squamous cell carcinoma (HNSCC) cell lines. It is suggested to be a promising agent in treating head and neck carcinoma owing to its anti‑proliferative and radiosensitizing characteristics. Moreover, it is suggested to have a radioprotective effect on salivary of rats subjected to total cranial gamma irradiation (Akyuz et al., 2017). It reduced the parameters of oxidative and nitrosative stress and acted as a free radical scavenger. Although this study lacks histological evaluation for confirming the results, it paves the way for studying this protective effect and its underlying molecular mechanism. Also, further studies are needed to determine if this effect is selective to normal tissues, but not tumor tissues. Proving this suggestion can lead to considering thymoquinone as an adjuvant for radiotherapy. Other suggested beneficial activities of thymoquinone include wound healing effect that may be conducted through the anti-inflammatory and anti-oxidant properties.
Thymoquinone is supposed to be a likely factor in treating allergic rhinitis, as it caused a reduction in allergic inflammation. It is supposed to be a promising coating material for mesh surface modification, as it has shown a reduction in adhesion formation, lymphocyte infiltration, polymorphonuclear leukocyte infiltration, granuloma formation, and capillary infiltration. The use of thymoquinone as an anticoagulant or as an adjuvant for the already existing anticoagulant and chemotherapy medications is under research. The antioxidant and anti-inflammatory activities of thymoquinone are thought to play a role in protecting the testes from the toxic effect of cadmium exposure. Thymoquinone is thought to protect against arsenic-induced testicular injury, as it ameliorated the testicular tissue injury, inhibited the decrease of serum testosterone, and decreased the elevation of testicular MDA and NO levels through decreasing the expression of inducible nitric oxide synthase and caspase-3. Also, it is stated to have a protective effect against testicular torsion.
Torsion causes ischaemia–reperfusion (I/R) injury and oxidative stress reactions; thymoquinone protects against this effect and reduces the apoptotic index. The same effect is applied to the case of ovarian torsion causing I/R injury. Thymoquinone is proved to have an inhibitory effect on benign prostatic hyperplasia (BPH) resulted from testosterone induction in Wistar rats. It caused a decrease in prostate weight/body weight ratio, a reduction in epithelial hyperplasia, expression of transforming growth factor-β1 (TGF-β1), expression of vascular endothelial growth factor-A (VEGF-A), and serum IL-6 level. Additionally, thymoquinone is reported to cause a regression in acute bacterial prostatitis (ABP) resulting from Pseudomonas Aeruginosa infection through the anti-oxidant and anti-inflammatory properties; this effect was proved by biochemical and histological findings. An experimental study was investigating the healing of left colon anastomosis. One of the major problems facing surgeons is the anastomotic leak. Thymoquinone increased the bursting strength of the anastomosis, the connective tissue production, and the tissue hydroxyproline levels. It reduced mucosal and submucosal damage and edema.
The anti-oxidant properties may be responsible for the suggested neuroprotective of thymoquinone in many neurodegenerative disorders such as Parkinson disease (PD). The attenuation of lipid peroxidation may cause neuroprotection against 6-hydroxydopamine (6-OHDA) induced neurotoxicity. Also, an experimental study stated that it could protect against pentylenetetrazole (PTZ) induced kindling and cognitive impairments in mice, and another one stated that it has a partial neuroprotective effect against acrylamide (ACR) induced neurotoxicity and gait abnormalities. On studying a rodent cerebral ischemia-reperfusion model, thymoquinone loaded PLGA-chitosan nanoparticles were administered through the nose to reach the brain.
They facilitated the delivery of thymoquinone to the brain tissue and could be used for the treatment of cerebral ischemia for their neuroprotective effect. Also, it exhibited anti-depressant effect in an animal model of depression through its anti-oxidant activity. In schizophrenia, it is supposed to have antipsychotic like action and to improve memory. On studying acoustic trauma-induced hearing loss, thymoquinone did not prevent the hearing loss, but if administered at a certain dose (20 mg/kg for 96 h), it may be able to repair the damage. In addition, thymoquinone is reported to protect against cisplatin-induced ototoxicity, as it preserved the thresholds of distortion product otoacoustic emissions (DPOAE) and auditory brainstem responses (ABR).
The Other Face of Thymoquinone
Despite all the studies conducted to prove the benefits of thymoquinone in different aspects, owing to its nature and ability to possess several mechanisms of action, it is still considered under investigations. It needs many experiments and clinical trials to verify its efficacy and understand its characteristics (Leung, Detremmerie, & Vanhoutte, 2017). Some previous studies investigated the effect of acute and sub-chronic toxicity of thymoquinone and demonstrated that it is well-tolerated. The acute oral toxicity showed some hypoactivity and difficulty in respiration, in addition to elevation of the serum level of variable enzymes such as lactate dehydrogenase (LDH) and creatine phosphokinase (CPK) (Leung et al., 2017). The sub-chronic toxicity showed a marked decrease in fasting plasma glucose level. On the other hand, intraperitoneal administration of thymoquinone resulted in peritonitis and hepatomegaly in some studies. On studying the effect of thymoquinone on augmentation of arterial contraction, it increased the intracellular levels of cyclic IMP that altered calcium handling and caused smooth muscle cell contractions. Thus, thymoquinone produced endothelium-dependent contractions, which were similar to hypoxic vasoconstrictions.
References
Abdel-Zaher, A. O., Mostafa, M. G., Farghly, H. M., Hamdy, M. M., Omran, G. A., & Al-Shaibani, N. K. (2013). Inhibition of brain oxidative stress and inducible nitric oxide synthase expression by thymoquinone attenuates the development of morphine tolerance and dependence in mice. European Journal of Pharmacology, 702(3), 62-70.
Agarwal, B., Campen, M. J., Channell, M. M., Wherry, S. J., Varamini, B., Davis, J. G., … Smoliga, J. M. (2013). Resveratrol for primary prevention of atherosclerosis: Clinical trial evidence for improved gene expression in vascular endothelium. International Journal of Cardiology, 166(1), 246-248.
Ahmed, A. M., Hersi, A., Mashhoud, W., Arafah, M. R., Abreu, P. C., Al Rowaily, M. A., … Al-Malah, M. H. (2017). Cardiovascular risk factors burden in Saudi Arabia: The Africa Middle East Cardiovascular Epidemiological (ACE) study. Journal of the Saudi Heart Association, 29, 235-243.
Akyuz, M., Taysi, S., Baysal, E., Demir, E., Alkis, H., Binici, H., … Karatas, Z. A. (2017). Radioprotective effect of thymoquinone on salivary gland of rats exposed to total cranial irradiation. Head & Neck, 39(10), 2027-2035.
Al-Ajlan, A. R. (2011). Lipid profile in relation to anthropometric measurements among college male students in Riyadh, Saudi Arabia: A cross-sectional study. International Journal of Biomedical Science, 7(2), 112–119.
Alkharfy, K. M., Ahmed, A., Khan, R. M., & Al-Shagha, W. M. (2015) Pharmacokinetic plasma behaviors of intravenous and oral bioavailability of thymoquinone in a rabbit model. European Journal of Drug Metabolism and Pharmacokinetics, 40(3), 319-323.
Al-Shehri, A. M. (2014). Prevalence and pattern of lipid disorders in Saudi patients with angiographically documented coronary artery disease. Journal of Family & Community Medicine, 21(3), 166–169.
Amin, B., & Hosseinzadeh, H. (2016). Black cumin (Nigella Sativa) and its active constituent, thymoquinone: An overview on the analgesic and anti-inflammatory effects. Planta Medica, 82(1), 8-16.
Babu, M., Nagaraja, B., & Reddy, K. B. (2016). Prevalence and distribution of atherosclerosis in different age groups in Tirupati area AP-Autopsy based study. The Pharma Innovation, 5(1), 87-91.
Chaieb, K., Kouidhi, B., Jrah, H., Mahdouani, K., & Bakhrouf, A. (2011). Antibacterial activity of Thymoquinone, an active principle of Nigella sativa and its potency to prevent bacterial biofilm formation. BMC Complementary and Alternative Medicine, 11(1), 29.
de Cássia da Silveira e Sá, R., Andrade, L. N., & de Sousa, D. P. (2013). A review on anti-inflammatory activity of monoterpenes. Molecules, 18(1), 1227-1254.
Farkhondeh, T., Samarghandian, S., & Borji, A. (2017). An overview on cardioprotective and anti-diabetic effects of thymoquinone. Asian Pacific Journal of Tropical Medicine, 10(9), 849–854.
Gali-Muhtasib, H., Fakhoury, I., Fatfat, M., Mismar, R., & Schneider-Stock, R. (2017). Combinatorial effects of thymoquinone on the anticancer activity of doxorubicin in adult T-cell leukemia. Cancer Research, 77(13), 1049.
Gholamnezhad, Z., Keyhanmanesh, R., & Boskabady, M. H. (2015). Anti-inflammatory, antioxidant, and immunomodulatory aspects of Nigella sativa for its preventive and bronchodilatory effects on obstructive respiratory diseases: A review of basic and clinical evidence. Journal of Functional Foods, 17, 910-927.
Glaser, J., & Holzgrabe, U. (2016). Focus on PAINS: False friends in the quest for selective anti-protozoal lead structures from nature? Medicinal Chemistry Communications, 7(2), 214-223.
Gore, P. R., Prajapati, C. P., Mahajan, U. B., Goyal, S. N., Belemkar, S., Ojha, S., … Patil, C. R. (2016). Protective effect of thymoquinone against cyclophosphamide-induced hemorrhagic cystitis through inhibiting DNA damage and upregulation of Nrf2 expression. International Journal of Biological Sciences, 12(8), 944–953.
Harzallah, H. J., Grayaa, R., Kharoubi, W., Maaloul, A., Hammami, M., & Mahjoub, T. (2012). Thymoquinone, the Nigella sativa bioactive compound, prevents circulatory oxidative stress caused by 1,2-dimethylhydrazine in erythrocyte during colon postinitiation carcinogenesis. Oxidative Medicine and Cellular Longevity, 2012(1), 1-4.
Herrington, W., Lacey, B., Sherliker, P., Armitage, J., & Lewington, S. (2016). Epidemiology of atherosclerosis and the potential to reduce the global burden of atherothrombotic disease. Circulation Research, 118(4), 535-546.
Hossen, M. J., Yang, W. S., Kim, D., Aravinthan, A., Kim, J. H., & Cho, J. Y. (2017). Thymoquinone: An IRAK1 inhibitor with in vivo and in vitro anti-inflammatory activities. Scientific Reports, 7(2), 1-12.
Husain, K., Hernandez, W., Ansari, R. A., & Ferder, L. (2015). Inflammation, oxidative stress and renin angiotensin system in atherosclerosis. World Journal of Biological Chemistry, 6(3), 209-217.
Inci, M., Davarci, M., Inci, M., Motor, S., Yalcinkaya, F., Sefil, N. K., … Zararsiz, I. (2013). Anti-inflammatory and antioxidant activity of thymoquinone in a rat model of acute bacterial prostatitis. Human & Experimental Toxicology, 32(4), 354-361.
Islam, M. T., Sultana, N., Riaz, T. A., Ferdous, J., Guha, B., Braga, A. L., … Cerqueira, G. S. (2016). Thymoquinone is knocking at the door of clinical trial. Internationl Archives of Medicine, 9(1), 1-14.
Ismail, M., Al-Naqeep, G., & Chan, K. W. (2010). Nigella Sativa thymoquinone-rich fraction greatly improves plasma antioxidant capacity and expression of antioxidant genes in hypercholesterolemic rats. Free Radical Biology and Medicine, 48(5), 664-672.
Khalife, R., Stephany, E., Tarras, O., Hodroj, M. H., & Rizk, S. (2014). Antiproliferative and proapoptotic effects of topotecan in combination with thymoquinone on acute myelogenous leukemia. Clinical Lymphoma Myeloma and Leukemia,14, 46-55.
Khan, M. A., Tania, M., Wei, C., Mei, Z., Fu, S., Xu, J., … Fu, J. (2015). Thymoquinone inhibits cancer metastasis by downregulating TWIST1 expression to reduce epithelial to mesenchymal transition. Oncotarget, 6(23), 19580–19591.
Kundu, J., Choi, B. Y., Jeong, C., Kundu, J. K., & Chun, K. (2014). Thymoquinone induces apoptosis in human colon cancer HCT116 cells through inactivation of STAT3 by blocking JAK2- and SRC‑mediated phosphorylation of EGF receptor tyrosine kinase. Oncology Reports, 32(2), 821-828.
Leung, S. W., Detremmerie, C. M., & Vanhoutte, P. M. (2017). O36 Endothelium-dependent contractions depending on biased sGC activation: The janus face of thymoquinone. Biochemical Pharmacology, 139, 121.
Libby, P. (2012). Inflammation in atherosclerosis. Arteriosclerosis,Thrombosis, and Vascular Biology, 32(9), 2045-2051.
Libby, P., Okamoto, Y., Rocha, V. Z., & Folco, E. (2010). Inflammation in atherosclerosis: Transition from theory to practice. Circulation Journal, 74(2), 213-220.
Lievens, D., & von Hundelshausen, P. (2011). Platelets in atherosclerosis. Thrombosis and Haemostasis, 106(5), 827-838.
Ma, Y., Yabluchanskiy, A., Hall, M. E., & Lindsey, M. L. (2014). Using plasma matrix metalloproteinase-9 and monocyte chemoattractant protein-1 to predict future cardiovascular events in subjects with carotid atherosclerosis. Atherosclerosis, 232(1), 231.
Mitchell, R. N. (2013). Learning from rejection: What transplantation teaches us about (other) vascular pathologies. Journal of Autoimmunity, 45, 80-89.
Nagi, M. N., Almakki, H. A., Sayed-Ahmed, M. M., & Al-Bekairi, A. M. (2010). Thymoquinone supplementation reverses acetaminophen-induced oxidative stress, nitric oxide production and energy decline in mice liver. Food and Chemical Toxicology, 48(9), 2361-2365.
Rabkin, S. W., Langer, A., Ur, E., Calciu, C. D., & Leiter, L. A. (2013). Inflammatory biomarkers CRP, MCP-1, serum amyloid alpha and interleukin-18 in patients with HTN and dyslipidemia: Impact of diabetes mellitus on metabolic syndrome and the effect of statin therapy. Hypertension Research, 36(6), 550-558.
Ragheb, A., Attia, A., Elbarbry, F., Prasad, K., & Shoker, A. (2011). Attenuated combined action of cyclosporine A and hyperlipidemia on atherogenesis in rabbits by thymoquinone. Evidence-Based Complementary and Alternative Medicine, 2011(2), 9.
Ragheb, A., Attia, A., Eldin, W. S., Elbarbry, F., Gazarin, S., & Shoker, A. (2009). The protective effect of thymoquinone, an anti-oxidant and antiinflammatory agent, against renal injury: A review. Saudi Journal of Kidney Diseases and Transplantation, 20(5), 741-752.
Ravindran, J., Nair, H. B., Sung, B., Prasad, S., Tekmal, R., & Aggarwal, B. (2010). Thymoquinone poly(lactide-co-glycolide) nanoparticles exhibit enhanced anti-proliferative, anti-inflammatory, and chemosensitization potential. Biochemical Pharmacology, 79(11), 1640-1647.
Rocha, V. Z., & Libby, P. (2009). Obesity, inflammation, and atherosclerosis. Nature Reviews Cardiology, 6(6), 399-409.
Shabana, A., El-Menyar, A., Asim, M., Al-Azzeh, H., & Al-Thani, H. (2013). Cardiovascular benefits of black cumin (Nigella sativa). Cardiovascular Toxicology, 13(1), 9-21.
Tabet, F., Vickers, K. C., Torres, L. F., Wiese, C. B., Shoucri, B. M., Lambert, G., … Barter, P. J. (2014). HDL-transferred microRNA-223 regulates ICAM-1 expression in endothelial cells. Nature Communications, 5, 1-14.
Woo, C. C., Kumar, A. P., Sethi, G., & Tan, K. H. (2012). Thymoquinone: Potential cure for inflammatory disorders and cancer. Biochemical Pharmacology, 83(4), 443-451.