Information about the Coronavirus
Coronaviruses are a well-studied group of viral particles containing RNA molecules as genetic material. The name of this family was given to viruses solely because of the similarity of their morphological structure to the solar corona. The lipoprotein envelope of the virus has several spike-like peplomers designed for efficient binding to cell receptors, as shown in Figure 1 below.
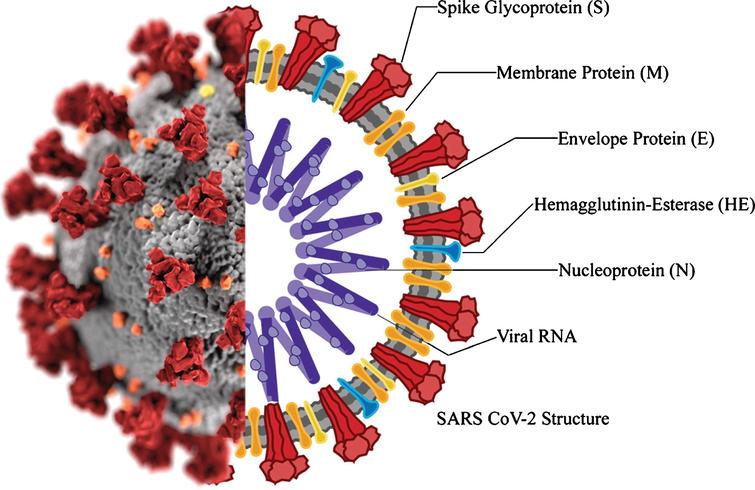
Morphological Characteristics
The morphological and genomic characteristics of the virus have already been sufficiently studied. According to Varga et al. (2020), the virions are roughly spherical or oval, with an effective diameter of about 180 nanometers. Morphological analysis of the pathogen shows the presence of multiple lipoprotein outgrowths of the viral envelope that fulfill the functional role of binding to target cell receptors and starting mechanisms to trigger infection. Thus, each peplomer consists of a protein S complex represented by three proteins. S1 at the top of the spike interacts with the target cell receptors, while S2 and S2‘ carry out a biochemical fusion of the viral envelope with the host cell membrane to facilitate the injection of genomic material (Varga et al., 2020). Figure 2 below shows different color codes for the varied types of proteins characterizing the virus.
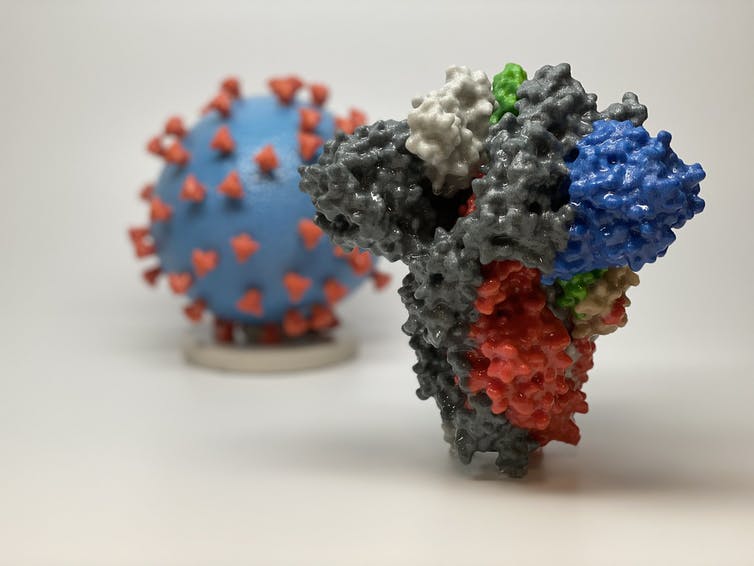
Figure 2 above contains images of the Delta virus model and its spike protein. The background image represents the virus model where the blue color denotes the surface area of the virus and the red color symbolizing the spike protein (Kindrachuk & Shaw, 2021). The foreground image provides a more detailed analysis of the spike protein that binds to the target cell receptors, thereby causing infections to the human cell. The key for the different colors of the spike protein appears in Table 1 below:
Table 1: Legend
As highlighted in Table 1 above, the black color of the spike protein signifies the receptor-binding protein, while the green color refers to the angiotensin converting receptor that is responsible for cell mutations. Alternatively, the white color represents the terminal domain that aids in cell binding. Broadly, these chemical characteristics of the spike protein and virus model described above are useful in understanding the biological characteristics of the virus and its epistemology (Kindrachuk & Shaw, 2021). These pieces of informational are relevant in providing clues about the rate of SARS-CoV-2 infections and the severity of illness.
Notably, each peplomer is generally mobile and is a hinge mechanism, which means that the attachment of SARS-CoV-2 to the cell surface proves to be more efficient for the virus. In addition to these proteins, the viral particle also has biopolymers that form the nucleocapsid envelope as shown in Figure 1 above. Such proteins package the viral RNA and play a fundamental role in the assembly of the viral particle, but the structure of the coronavirus nucleocapsid is not currently described in any detail (Covián et al., 2020). From the known information, it should be noted that the internal proteins of the capsid are bound in a chain no thicker than 15 nanometers, with the capsid as a whole not occupying the entire free volume of the pathogenic particle. Inside the nucleocapsid, as is customary for viruses, there is genomic material.
Genomic Characteristics
SARS-CoV-2 is a typical RNA-containing coronavirus with reliable morphological evidence of belonging to this taxon. The virus contains a single-stranded RNA measuring approximately 29.8 base pairs (Rokni et al., 2020). The genome itself has six open reading frames, which are characteristic of all coronaviruses and genes unique to SARS-CoV-2 responsible for synthesizing matrix and nucleocapsid proteins. SARS-CoV-2 should be assigned to the beta-coronaviruses (Coronaviridae family) among which there is SARS-CoV, which caused SARS pneumonia. This allows the current pathogen to be evaluated as closely related to preexisting forms but mutated and has crossed the interspecies barrier.
Aetiological Evidence
SARS-CoV-2 is a pathogen of the respiratory system of humans. The virus is capable of causing acute respiratory viral infections, including respiratory failure (Xia et al., 2021). Figure 3 below is a sample of a functional and infected lung.
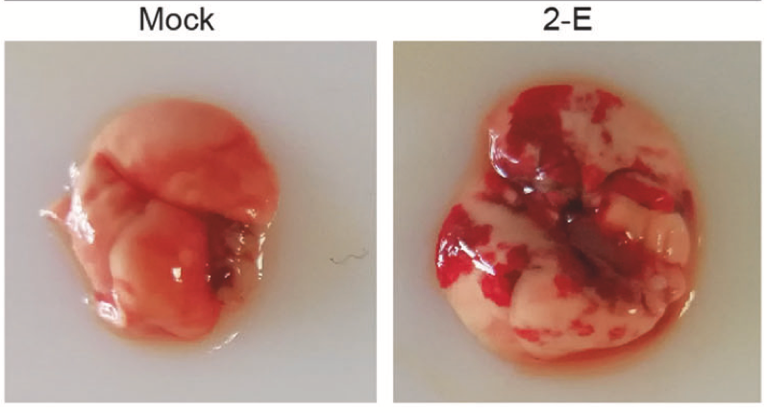
Figure 3 above contains two images of the “Mock” and “2-E” samples of a mouse’s lung. The “Mock” image represents a picture of a healthy lung, while “2-E” symbolizes one where infection has occurred (Xia et al., 2021). Image 2-E is associated with microphage infiltration and cytokine production, as would be the case in the human lung if infection occurred. This image was reproduced after the lung was transfected with 2-E plasmids for a maximum of 48 hours (Xia et al., 2021). The outcome was a secondary inflammation cascade, signifying damage to the host cell. Therefore, it is believed that microphage infiltration in image 2-E causes ARDS-like pathological damages to the lung (Xia et al., 2021). Given that infection could occur rapidly due to microphage infiltration in image 2-E, multiple organ failure could be experienced.
References
Kindrachuk, J., & Shaw, S. (2021). COVID-19 Delta variant in Canada: FAQ on origins, hotspots and vaccine protection. Web.
Xia, B., Shen, X., He, Y., Pan, X., Liu, F. L., Wang, Y.,… & Gao, Z. (2021). SARS-CoV-2 envelope protein causes acute respiratory distress syndrome (ARDS)-like pathological damages and constitutes an antiviral target. Cell Research, 31(1), 1-14. Web.