Introduction
The cellular fluid makes up total body water in multi-cellular organisms. It is divided into two distinct compartments: extracellular and intracellular environments. The latter (ICF) is localized inside cells and accounts for about two-thirds of cellular fluid (Marieb and Hoehn 143). The remainder, which constitutes one-third of total body water, occurs outside cells as extracellular fluid (ECF). Electrolyte concentrations in each compartment affect cell and tissue function, and thus, they are under homeostatic regulation. Homeostasis controls ionic levels (Na+, K+, Ca+, etc.) and pH as well as ECF and ICF volumes. This paper describes the components of a cellular fluid and regulatory mechanisms for achieving electrolyte balance in the ICF and ECF.
Discussion
Biochemical reactions in cells can only occur in an aqueous medium. Water containing electrolytes, e.g., Na+, diffuses across semi-permeable membranes along an osmotic gradient (Voet et al. 78). Therefore, differences in solute concentration would lead to water movement between compartments in response to osmotic pressure. An electrolyte balance of solutes between extracellular and intracellular environments is required for normal tissue function. The cellular fluid is the medium for transporting dissolved solutes, nutrients, and gases and proteins between the cytoplasm and interstitial spaces (Voet et al. 78). Therefore, it controls electrolyte concentrations in the body through these exchanges.
Cellular fluids occur in compartments differentiated by a natural barrier, i.e., the plasma membrane, as shown in the image below (Figure 1). This distinction is only superficial as the two parts are mostly similar in composition. The ICF comprises the fluid within the cells, and it is the primary constituent of the cytosol. It accounts for 60% of total body water in humans, estimated to be 25L in a mature male or 40% of body weight (Marieb and Hoehn 147). The volume of ICF is less prone to changes due to the electrolyte balance maintained through homeostatic regulation. A decrease in water content in the ICF would increase solute concentrations and interrupt normal cell functioning, while excess solvent would cause osmotic lysis.
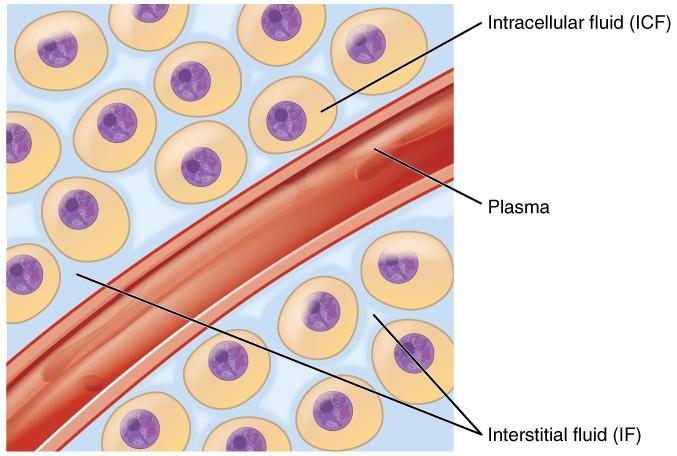
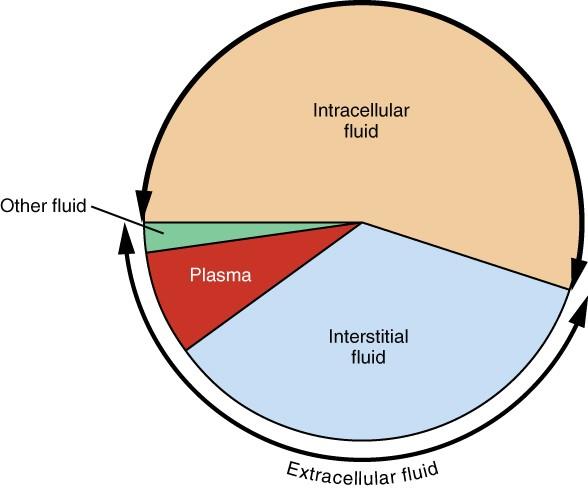
About one-third of body fluids or 20% of body weight is ECF (Marieb and Hoehn 151). It comprises plasma and interstitial fluid (80%) that occurs in the intercellular spaces, as shown in Figure 1. The two components have different roles. Plasma moves through the circulatory system carrying with it solutes, nutrients, hormones, CO2, O2, metabolic wastes, etc. (Marieb and Hoehn 153). These substances pass through capillaries into the interstitial fluid (IF) that bathes and nourishes the cells. A semi-permeable membrane separates the ICF and the IF. In addition to plasma and IF, the ECF consists of the “cerebrospinal fluid in the central nervous system, synovial fluid, pleural fluid, pericardial fluid, and peritoneal fluid” (Marieb and Hoehn 153). The specific proportions of each fluid in the body are represented in Figure 2 below.
The compartments have different electrolyte profiles; however, all ECF fluids have primarily similar components. The dominant cation and anion in plasma are Na+ and Cl–, respectively (Widmaier et al. 63). It also contains high levels of bicarbonate and soluble proteins. The interstitial fluid resembles plasma in composition, but it has lower peptide concentrations (Widmaier et al. 65). The ICF contains K+, PO42-, Mg2+, and proteins at high levels. In general, the electrolytes occurring in large amounts in the ECF (plasma) are Na+ and Cl– ions, while the primary cation and anion in ICF are K+ and PO42-, respectively.
Cellular fluids are chemically inactive since the anionic-cationic balance is always maintained. Thus, the net charge in ICF and ECF is zero; however, potassium or sodium ions may sometimes escape into or out of the intracellular fluid or cytosol. This electrolytic imbalance is corrected through the sodium-potassium pumping system that actively transports Na+ out of the ICF and K+ into the cell (Voet et al. 78). The movement of the nutrient-rich plasma into the interstitial fluid is due to the hydrostatic pressure generated in the capillaries. However, on the venous side, this force is lower, which allows IF to drain back into the blood. The excess fluid ends up in the lymphatic system.
Solutes move between ICF and ECF through active transport. Through this process, the ionic movement across the cell membrane can occur against the concentration gradient using ATP (Widmaier et al. 62). The sodium-potassium pump actively moves ‘leaked’ Na+ and K+ to maintain the electrolyte balance. The movement of ions may also involve a passive process. In this case, molecules would migrate from a high-concentrated area to a low-concentrated medium across a membrane (Widmaier et al. 62). Water primarily moves between compartments through osmosis and membrane-bound aquaporins. Protein channels, such as glucose transporters, facilitate the movement of molecules without using energy.
The regulation of cellular fluids is crucial to maintaining the osmotic pressure required for normal tissue functioning. A rise or drop in extracellular osmolarity affects cytosol volume, resulting in impaired cell functioning. Thus, water input must equal output (urine, sweat, etc.) to establish this balance. An increase in plasma osmolarity triggers the release of the antidiuretic hormone (ADH) by the posterior pituitary (De Luca et al. 34). Its effects include antidiuresis (low urine output) in the kidneys and vascular constriction to increase capillary pressure. Intense perspiration, vomiting, diarrhea, and baroreceptors in the vessels can also trigger ADH release (De Luca et al. 35). This hormone reduces urinary output by increasing water reabsorption from the glomerular filtrate.
A decrease in osmolarity leads to ADH inhibition and thirst suppression to control cellular hydration. The conditions such as edema and water intoxication may cause water imbalance in the ICF and ECF. Hypotonic hydration may also result from renal insufficiency that reduces the urinary output rate. A drop in ECF osmolarity causes hyponatremia, which is characterized by a net water movement into the cells, resulting in swelling. The edema manifests as an excessive accumulation of IF that leads to tissue swelling (De Luca et al. 41). It occurs when too much fluid filters out of the vascular system into the intercellular spaces due to elevated hydrostatic pressure or enhanced capillary permeability. It causes nausea, cramping, and cerebral dropsy, which may be fatal.
The regulation of sodium balance also affects ECF and ICF volumes. Aldosterone is the hormone that controls Na+ and K+ in the fluids; it reabsorbs these ions in the kidney tubes, resulting in reduced urinary output (Palmer 1054). The regulatory mechanism involves the rennin-angiotensin-aldosterone pathway, whereby sympathetic nervous stimulation triggers the release of rennin that induces the formation of angiotensin II, which prompts aldosterone secretion (Palmer 1054). As a result, the reabsorption rate increases to bring about water balance in ECF and ICF.
Conclusion
The cellular fluid is compartmentalized into two parts: ICF and ECF that comprises plasma and IF, among others. Electrolyte concentration and water balance are required in these compartments for normal tissue function. Regulatory mechanisms involving hormones such as ADH and aldosterone help maintain the essential osmolarity through solute and water reabsorption by the kidney tubules.
Works Cited
De Luca, Antonio, et al. Neurobiology of Body Fluid Homeostasis: Transduction and Integration. CRC Press, 2013.
Marieb, Elaine, and Katja Hoehn. Human Anatomy and Physiology. 9th ed., Pearson Education, 2013.
Palmer, Biff F. “Regulation of Potassium Homeostasis.” Clinical Journal of the American Society of Nephrology, vol. 10, no. 6, 2015, pp. 1050-1060.
Widmaier, Eric, et al. Vander’s Human Physiology: The Mechanisms of Body Function. 13th ed., McGraw-Hill Education, 2013.
Voet, Donald, et al. Fundamentals of Biochemistry: Life at the Molecular Level. 5th ed., John Wiley & Sons, 2016.