In the academic environment of chemical processes, named reactions are of particular importance because they are of applied value or solve problems of intergroup transition between compounds. Among the named processes, the Diels-Alder reaction, or diene synthesis, traditionally used to produce stereospecific six-membered carbon cycles with multiple bonds, is of excellent research popularity. Two types of reagents are used for this reaction, one being a conjugated diene and the other a dienophile. As a dienophile can be used, any substances with π-bond, including alkenes or acetylenes with activating electron acceptor groups, and diene must meet the requirements of chemical stability, conjugation of bonds, and the ability to take s-cis configuration. When they interact, the electron density is redistributed with the formation of single multiple bonds with a six-membered cycle.
The double name of this reaction is due to the history of its creation. In 1927, the process was patented by German organic chemists Otto Diels and Kurt Alder (Diels & Alder, 1927). Since the synthesis of six-membered homocyclic has applications in organic chemistry, including the possibility of using them to produce aromatic rings, Diels and Alder were awarded the Nobel Prize for their development in 1950.
Since then, the possibilities of diene synthesis have expanded significantly; this includes the possibility of obtaining bicyclic compounds if cyclic dienes were used as raw materials. However, the basis has remained unchanged; namely, the addition of a dienophile to a diene by 2,4-conjugation allows the preparation of non-aromatic homocyclic with one multiple bonds in them. Initially, an intermediate compound with unstably separated multiple bonds is formed, which stabilizes over time into a stable homocycle. The exothermic reaction is carried out without additional reagents in an inert solvent at low temperatures, which is an additional advantage in terms of its practical implementation in the laboratory.
Procedure
For the present diene synthesis reaction, butadiene sulfone was used to produce a conjugated diene system when heated. The use of butadiene-1,3 instead of butadiene sulfone was complicated by the gaseous state of the diene, which made the process technically challenging to implement. Thermal decomposition of solid butadiene sulfone was carried out to produce toxic sulfur dioxide and butadiene-1,3. The sulfur dioxide was captured with water since using only a fume hood would result in air contamination.
Thus, 2.29 g of butadiene sulfone was added to 1.22 g of finely dispersed maleic anhydride in a heat-resistant 50-milliliter flask. Both substances were dissolved in inert xylene (3.0 mL), and a boiling stone was used to stabilize the boiling process. A reflux condenser was hermetically attached to the opening of the flask, and all potential gaps were sealed with a rubber baffle on top, according to Figure 1 below. Several wet paper towels were additionally attached to the beaker to trap the toxic sulfur dioxide vapors.
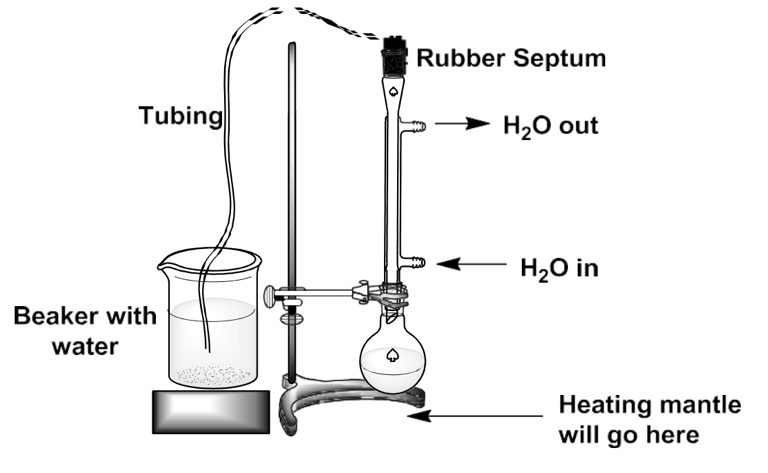
The installed reflux system was slowly heated for 30 minutes, and the cyclohex-4-ene-cis-1,2-dicarboxylic anhydride released during this process remained in the round-bottom flask. After a five-minute cooling down after the completion of heating, the process was repeated but with 5 mL of distilled water added to the round-bottom flask. After 30 minutes of repeated heating, the mixture was cooled, and the synthesized crystals were collected by vacuum filtration with 2×2 portions of ice water. The cyclohex-4-ene-cis-1,2-dicarboxylic acid crystals were dried and analytically weighed to determine their exact mass for further analysis. If the crystals were not distinguishable after the reheating procedure, the addition of a few drops of concentrated sulfuric acid should have catalyzed the process.
Reference
O. Diels and K. Alder, Brit. Pat. 300130 (1927).