Introduction
There are several ways in which food may be preserved today. Traditionally, drying and smoking were the most common methods of preserving food (Thorne, 1996). Today, more complex methods such as the use of radiations and chemicals exist. There are five common ways that food can be preserved today. The aseptic method of preservation removes and prevents the entry of undesirable micro-organisms into food by application of the highest level of hygiene standards. The hygiene standards are enhanced in all areas that come into contact with food such as utensils and food storage. Other similar methods of preserving food are sterilization, freezing, drying, and refrigeration (Lichtenberg, 2002).
In addition, foods may also be conserved using some harmless chemicals. The addition of some non-toxic chemicals in food hinders the growth of harmful microorganisms. Usually, the chemicals inhibit the rate at which energy may be released by the cells of the microorganisms. There are several chemical food preservatives such as nitrates, organic acids and sulfur dioxide. This paper covers microbial preservation of food using chemicals. The paper will cover different chemicals and how they are able to explicitly preserve food. In addition, microorganisms that are resistant as well as those which are very sensitive to such chemicals will also be discussed. Lastly, the paper will detail how every chemical should be prescribed during the food processing process and the necessary safety checks that should be observed.
Nitrites
Nitrites are used as food preservatives. Usually, they are used in red meat and fish preservation. At times, they may also be used in preserving poultry products. Some nitrates are also used for flavoring foods, especially beef products. Nitrites preserve food by inhibiting the spread and multiplication of Clostridium botulinum bacteria. This bacterium is very toxic when present in any food due to its chemical compound neurotoxin. This toxin makes a human being who has consumed it exhibits symptoms such as those of an individual suffering from paralysis (U.S. Department of Agriculture, 1994). Nitrites inhibit the growth of these bacteria by obstructing the respiratory chains and genetic links.
Nitrites react with myoglobin, a protein that is common in foods rich in protein. When this compound reacts with myoglobin that is found in blood’s hemoglobin, the product is a stable compound called nitric oxide myoglobin. Nitrites are responsible for preserving most of the meats and avoiding meat from turning brown. When meat is cooked, the myoglobin oxide is usually converted into a non-toxic compound called nitrosohemochrome which is stable (Jay, 2005).
However, one problem with the use of nitrites as preservatives is that they react with some amino acids. When this happens, the compound formed is known as nitrosamine which is believed to be one of the causes of stomach cancer (Thorne, 1996). Usually, this chemical is used in the preservation of meat. It preserves color and meat freshness. It is common in the form of powder and white in color. Nitrites especially those of calcium are used by plants for enhancing growth. The chemical is still considered not to be a hazard in the amounts that people are currently exposed to (Thorne, 1996).
When people eat vegetables and processed foods such as canned beef, nitrates often get into our bodies. Human beings are able to convert nitrates into common foods. This form which is converted by the body is called endogenous nitrite. Food additives have permitted levels of nitrite which is of external origin (exogenous). Nitrite reacts or interacts with hemoglobin and may cause poor blood flow and hence poor oxygen supply in the body. For infants, the reaction of hemoglobin and nitrite may result in a condition known as methaemoglobinaemia. Nitrites are not recommended for young children. This is more serious for infants who are below the age of three months (Roberts, 2001). The Acceptable Daily Intake (ADI) for nitrite is very important and should be observed to ensure healthy conditions. A good balance should be established for exogenous and endogenous nitrite towards establishing the ADI. The ADI for nitrite in a normal person should be 0.07mg for every kilogram of body weight every day (FSANZ, 2010).
Sulfur Dioxide
Preservatives are conveniently placed in three groups; antioxidants which inhibit the oxidation of lipids in food causing rancidity, antimicrobials which slow down the rate of growth of bacteria and those that entirely block any enzymatic processes in food hence stopping any microbial growth. Sulfur dioxide is considered to possess all three attributes mentioned above. This explains why sulfur dioxide and its related compounds are often and commonly used in many household products (Gould, 2000).
Sulfites in food react with cell currency, adenosine triphosphate and hinder the path for any reactions. The blockage of any metabolic pathways and hence the cellular transport hinders the growth of microorganisms responsible for food spoilage. The chemical usually inhibits the growth of fungi and other bacteria. Pathogens are usually inhibited from multiplying by the acidity found in this chemical (Roberts, 2001).
Sulfur dioxide is a major contributor to keeping many fruits fresh through its ability to block enzymatic browning. This reaction known as Maillard helps in the reduction or amino acids and some sugars hence inhibiting spoilage. The recommended or acceptable daily Intake for sulfur dioxide is 7mg/kg of body weight every day (FDA, 2010). Care should be taken to avoid excessive consumption of this compound since it may cause inflammation and stomach irritation. In addition, excess sulfites may also lead to severe headaches. Sulfites are commonly used in fresh vegetables. It is also an excellent preserver of fruits (Russel, 2003).
Organic Acids
Sorbic acid
Sorbic acid usually keeps the pH levels in food to be preserved very low. In such acidic environments, enzymes are unable to multiply. This inhibits phenolase and hence keeps the preserved food fresh. Sorbic acid is a fatty acid with a straight chain structure. The acid is relatively soluble at normal room temperature. Most sorbates such as those of sodium are stable in their solid form but are quite unstable in the solution state due to their ability to dissociate on oxidation. This acid is very valuable in the fight against molds and yeast. They are used in carbonated drinks and some fruit products. The ADI for Sorbic acid is in the range of 0.05 to 0.3 percent (FSANZ, 2010). This preservative does not in any way affect food quality and flavor.
Propionic Acid
Propionic acid is commonly used due to its effective action against fungal. The acid is naturally found in sweat and in reactions that involve bacterial fermentation. This acid is very effective in fighting the growth and multiplication of microorganisms. Usually, the acid is effective in fighting molds. When favorable environments, propionic acid is known to effectively fight bacteria. Usually, the acid is used in cow feeds. Naturally, this acid may be found in grains. Occasionally, it may also be found in some fruits such as strawberries. This acid is known for its effective microbial work in fighting bacillus mesentericus. The preservative also acts as an inhibitor to organisms that cause milk fever disease in cows. This acid is generally accepted in the European region for the uses named above. In addition, it is used in the preservation of other foods such as canned fruits where molds are likely to infest (Jay, 2005).
This acid works by ensuring that cells are not able to produce energy which is necessary for the growth of a microorganism. This means that the bacteria are inhibited from feeding or affecting their enzymes for multiplication and survival. The recommended food additive for this acid is 0.1 to 0.4 for foods meant for human consumption but can be as high as 1% for foods meant for animals such as cows (FDA, 2010). Care should be taken when adding this preservative since it is toxic at high concentrations. At high concentrations, this acid may lead to allergies and migraines especially among patients who have had the same before. However, the body is able to break this acid into fatty acid which is not stored in the body (Rosenthal, 1992).
Parabens
Parabens are very similar to benzoic acid in the way they react with microorganisms. However, this acid is more effective than benzoic acid at higher pH values. Parabens are close in working method to benzoic acid only that they work well in both the alkaline and the acid regions as antimicrobials. They have antimicrobial effects that are proportional to their alkyl groups. They are very good at controlling yeast and molds. This acid is less efficient against destroying bacteria (Fellows, 2009). Parabens are commonly used in the preservation of vegetables such as ketchup and fish. Sometimes the parabens are used in preserving drugs.
In addition, this chemical is effective in fighting gram-positive bacteria. However, it may not be very strong or effective in the fight against gram-negative bacteria. Parabens are commonly used in preserving pastries and fruit cakes. The recommended or acceptable intake level should be between 0.1 to 0.5 mg/kg of body weight in a normal human being every day (FSANZ, 2010). When used in excess, parabens may lead to stomach-related problems such as inflammation. In some cases, they have been observed to cause irritation in the stomach when used in excess.
Benzoic Acid
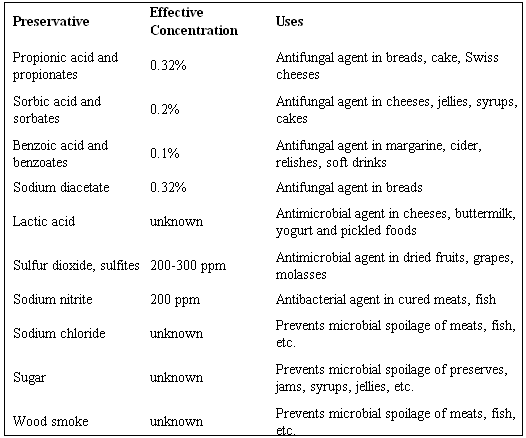
Benzoic acid may be found in its natural form in some fruits such as prunes, plums and some other spices. As a preservative, benzoic acid may be used in its two most common forms; benzoate and benzoic acid (Townsend, 2007). However, benzoate is more miscible with water and hence more commonly utilized. The optimum range of use of benzoic acid as a preservative is a pH of between 2.5 and 4.0. This mild acidity in benzoate makes the growth of microorganisms to be retarded hence preserving food. Plants use this acid especially in the fruits section to enhance ripening. Benzoic acid should be taken in the recommended 0.1 mg/kg every day and should not be exceeded since it is harmful to the body. Todar (2008) confirms that the acid may cause inflammations if used for a prolonged period and may also cause some poor cell growth in infants. The table in appendix 1 shows some of the acids discussed above with their recommended concentrations (Todar, 2008).
Acids Used as Disinfectant Sprays
The most common acid used as a disinfectant spray or dip in foods is peracetic acid. Disinfecting acids have a similar way of fighting microorganisms in present food. For instance, peracetic acid is a strong oxidizing agent (Russel, 2003). This acid destroys the action of a microorganism by oxidizing the cell covering. The oxidation process in peracetic acid involves the transfer of electrons which when transferred to the microorganism makes it inactive and as a result dies. This acid is effective at pH levels of about 2.8. This acid is dangerous when used in large quantities as a preservative. However, its ease of dissociation into oxygen, water and carbon dioxide which are all non-toxic compounds makes this acid friendly (Roberts, 2001).
This acid easily dissolves in water. This means that the body can easily dissolve the acid when used in foods. This acid fights fungi and bacteria effectively, especially from fresh vegetables. In addition, it may be used in water purification since it has the ability to remove Legionella bacteria. At certain temperatures and pH levels, this acid can also be used to fight viruses and algae.
Compounds Used for Disinfection of Water
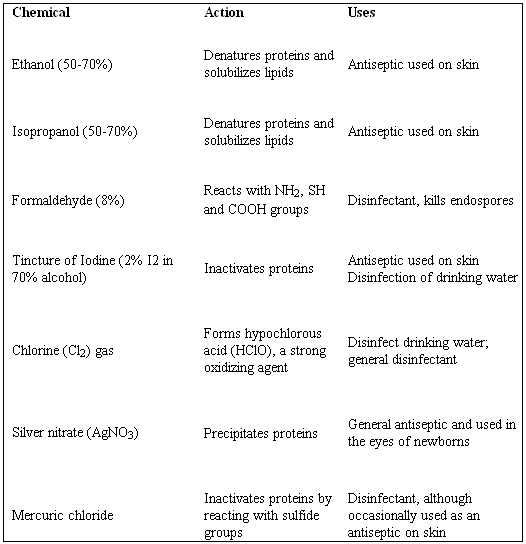
Disinfectants refer to chemical agents used in controlling the growth of microbial. They differ from other chemicals in that they do not necessarily kill the spores of the microorganisms. These agents, therefore, kill the microorganisms but they are not safe when used on living tissues. (Montville, 2008). This, therefore, leaves them to be used on tools that do not contain living tissues such as tables, utensils and floor. Examples of such chemical compounds are chlorine, hypochlorite, ammonium compounds, phenolics and formaldehyde. Disinfectants differ slightly from antiseptics. According to Todar (2008), antiseptics are a bit mild such that they may be exposed to living tissues but may not be used internally. Examples are such as alcohols, iodine solution and some detergents as shown in appendix 2.
Usually, disinfectants are gauged by their ability to react with the mucous membrane which usually depends on their concentration. The harmlessness of these disinfectants usually depends on the available amount of acid in the solution. For instance, chlorine is one of the most common disinfectants (Borgstrom, 1996). This element disinfects water by creating hypochlorous acid which is a powerful oxidizing agent. This mild acidity and oxidizing effect of chlorine in water inhibit the development of microorganisms. Chlorine is also used in water to fight to scale of water and hence comes in s an ingredient in water. About sixteen drops of chlorine inside three-quarters of a gallon are enough (Shibasaki, 1992). Excessive use of chlorine is harmful to one’s health. When chlorine is used in excess, it may react with natural organic compounds to form trihalomethanes (THM). Chlorine is limited in efficiency against protozoa despite being strong on bacteria. The use of clean and disinfected water is important when preparing drinks. This chemical is able to kill most disease-causing microorganisms in the water meant for preparing drinks and for cooking (EPA, 2010).
Conclusion
Currently, food scientists are looking for new and natural ways of preserving food without excessive use of chemicals which occasionally have negative effects on the body. Due to the ease of reaction of chemicals with other chemicals that a person consumes, this method of preserving food needs to be conducted with a lot of care to ensure that people are free from danger (Bender, 1995). When choosing the type of preservative to be used for a particular food, it is important to consider some factors. The antimicrobial features of a preservative are very important since they give an idea of how the results will be achieved against certain microorganisms found in food. The expected age group that the food is meant for also helps in choosing specific chemicals which may be used. It is important to observe all safety and ADI procedural requirements for any food preservative to ensure that foods remain healthy for consumption.
References
Bender, A. (1995). A dictionary of food and nutrition. London: Oxford University Press.
Borgstrom, G. (1996). Principles of food science. New York: Routledge Publishers.
EPA. (2010). Emergency disinfection of drinking water. Web.
FDA. (2010). Kinetics of microbial inactivation for alternative food processing technologies. Web.
Fellows, P. (2009). Food processing technology: Principles and practice. London: CRC Press.
FSANZ. (2010). Food additives. Web.
Gould, W. (2000). Innovations in food processing. New York: CRC Press.
Jay, M. (2005). Modern food microbiology. New York: Springer.
Lichtenberg, E. (2002). Storage technology and the environment. Journal of Agricultural and Resource Economics, 27, 104-1118.
Montville, T. (2008). Food microbiology: An introduction. New Jersey: ASM Press.
Roberts, C. (2001). The food safety information handbook. New York: Oryx Press.
Rosenthal, I. (1992). Electromagnetic radiation in food science. New York: Springer-Verlag.
Russel, J. (2003). Food preservatives. Washington: Springer.
Shibasaki, I. (1992). Food preservation with nontraditional antimicrobial agents. Journal of Food Safety, 4(1), 35-58.
Todar, K. (2008). Control of microbial growth. Web.
Townsend, M. (2007). Teaching the nitrogen cycle and human health interactions. Journal of Geoscience Education, 55, 56-102.
Thorne, S. (1996). The history of food preservation. Totowa, NJ: Barnes & Noble Books.
U.S. Department of Agriculture. (1994). Complete guide to home canning. Washington, DC: U.S. Government Printing Office.
Appendices