Introduction
The purpose of the experiment was to examine the expansion of a perfect gas by determining the specific heat ratio through an adiabatic process and the ratio of volume using an isothermal process. The experimental setup included two vessels (a small vessel and a large one), a top plate, a common base plate, an air pump, two temperature sensors, seven valves, and two pressure sensors. Sensor P measured the air pressure in the large vessel, whereas sensor V determined the vacuum pressure in the smaller vessel.
Methodology
The expansion process of a perfect gas was examined experimentally through two experiments. The first part involved determining the heat capacity ratio through an adiabatic reversible expansion to an intermediate pressure and returning the temperature to the initial value at constant volume.
The heat capacity ratio was determined by first measuring and recording the atmospheric pressure (Patm). Ball valves V1 and V3 were closed, whereas valve V4 was opened. The large vessel was pressurized by switching on the air pump until the pressure reached 30 kN/m2. At that point, the air pump was switched off, followed by the closing of valve V4. The starting pressure Ps was recorded after the pressure in the large vessel had stabilized. Valve V1 was then opened quickly leading to the escape of a small quantity of air. The instantaneous pressure Pi was recorded as well as the final pressure Pf when the vessel returned to ambient temperature. The procedure was repeated at varying pressures with the final pressure of the previous experiment being used as the starting pressure of the subsequent run. The respective values were recorded in Table 1.
For the second part of the experiment, one vessel was pressurized and left to return to stability at ambient temperature. This process was achieved at constant pressure by permitting the leakage of air from the pressurized container through a needle valve to a different sized vessel. The ratio of the volumes of the vessels was computed by monitoring the pressure before and after the procedure. The observed volumes were recorded in Table 2.
Results
Test A
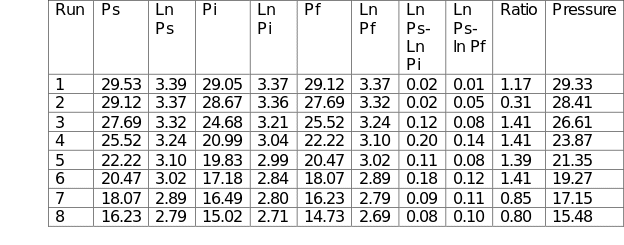
The experimental data were recorded in Table 1 below. The capacity ratio was calculated in Microsoft Excel using the following formula:
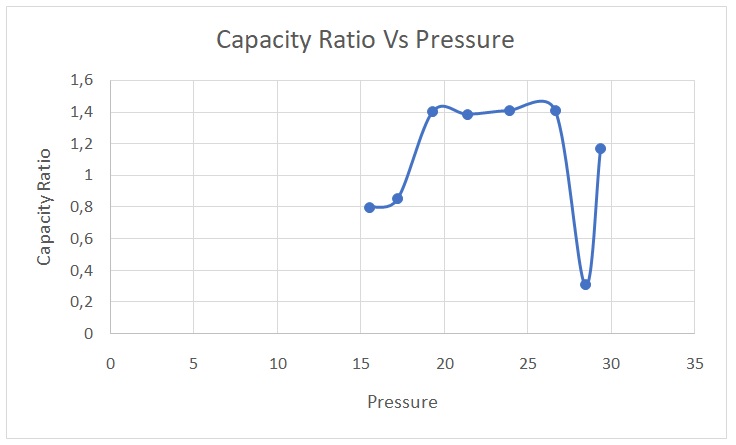
γ = Cp/Cv = Ln (Ps) – Ln (Pi)/ Ln (Ps) – Ln (Pf). The data were then used to plot Figure 1. The average capacity ratio for the entire process was 1.0932.
Test B
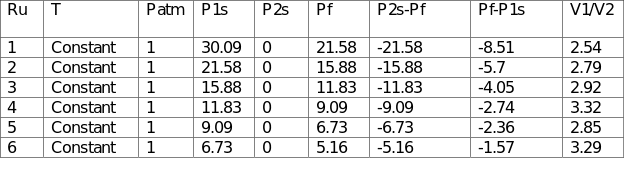
The resultant pressures were recorded in Table 2 as shown below. The volume ratio of the vessels was determined using the equation:
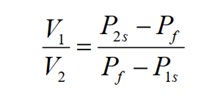
The average ratio of V1/V2 was 2.95, which implied that the large vessel was approximately three times bigger than the small vessel.
Discussion
The average experimental value of the capacity ratio (1.0932) differed from the theoretical value of 1.4. This disparity could be attributed to experimental errors during certain trials. However, the experimental values matched the theoretical ones for trials 3,4,5, and 6. In adiabatic processes, there is no conveyance of mass or heat between a system and its surroundings (Serway & Jewett 2018). Compressing a gas adiabatically means that work is done on the gas, which leads to an increase in temperature. Conversely, expanding a gas adiabatically lowers its temperature. The initial expansion process can be considered adiabatic because the gas did the work during the stabilization of the pressure in the vessel, leading to a decline in temperature.
For Test B, the outcomes (2.95) differed slightly from the expected ones given that the actual V1/V2 was 2.46. The air in the vessels behaved like a perfect gas during the procedure. However, the disparities could be attributed to errors such as leakage of air, resulting in lower pressures and larger projected volumes. The rate of change does not affect the temperature of the air inside the vessels because the process was isothermal.
Conclusion
Ideal gases obey all the gas laws at respective volumes, temperatures, and pressure. However, in real-life scenarios, gases tend to deviate from the behavior of perfect gases. This deviation was responsible for the observed differences from expected (theoretical values) and experimental values.
Reference List
Serway, RA & Jewett JW 2018, Physics for scientists and engineers with modern physics,10th edn, Cengage Learning, Boston, MA.