Introduction
Chromatography is an analytical chemistry technique used to separate a blend of substances into their constituents. The fundamental principle of all forms of chromatography is that the mobile phase carries the constituents of the analyte through the stationary phase by capillary action. Various compounds travel at varying rates. The rate of migration relies on the strength of the chemical interactions between the analyte and the mobile phase as well as the interactions between the analyte and the stationary phase.
Paper chromatography uses homogeneous porous paper as the stationary phase (David and Underwood 209). The mobile phase is usually an appropriate solvent or a blend of several solvents. The proportion of the distance covered by the substance to the distance traveled by the solvent is what is referred to as the ratio-to-front (Rf) value. The Rf value is constant for a specific compound provided all other factors (such as solvent components and paper type) are kept constant.
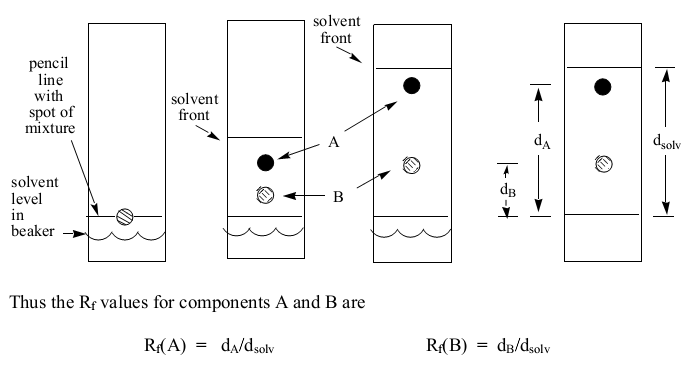
It is possible to identify compounds using their Rf values since similar compounds have the same Rf value at similar conditions. Alternatively, a compound can be detected by matching the location of its spot with the location of a similar spot of a recognized substance. However, in some instances such as amino acids, the spots can be colorless. Such cases require that the spots are reacted with a substance that yields a colored product such as ninhydrin.
Paper chromatography has numerous applications such as establishing the presence of the desired compound in a mixture of substances or determining the effectiveness of purification processes. This is especially useful in determining pollutants in environmental analysis, food analysis, as well as in cosmetology. Identification of amino acids is also important in recognizing inborn errors of metabolism. The advantages of paper chromatography are that it is fast and cheap.
Experimental Section
A Whatman No. 1 filter paper was cut into a rectangular shape. Five amino acids cysteine, leucine, glycine, arginine, and alanine were used at the standards in the identification of two unknown amino acids unknown #3 and unknown #1. A pencil was used to draw the baseline on the filter paper after which drops of the test samples and the standards were applied repeatedly on the baseline. Each spot was then labeled accordingly. The paper was appended in a beaker holding a low level of the solvent with the lower portion of the paper (just before the baseline) touching the solvent. This was carefully done so that the spots were not immersed in the solvent. The beaker was then covered using a watch glass.
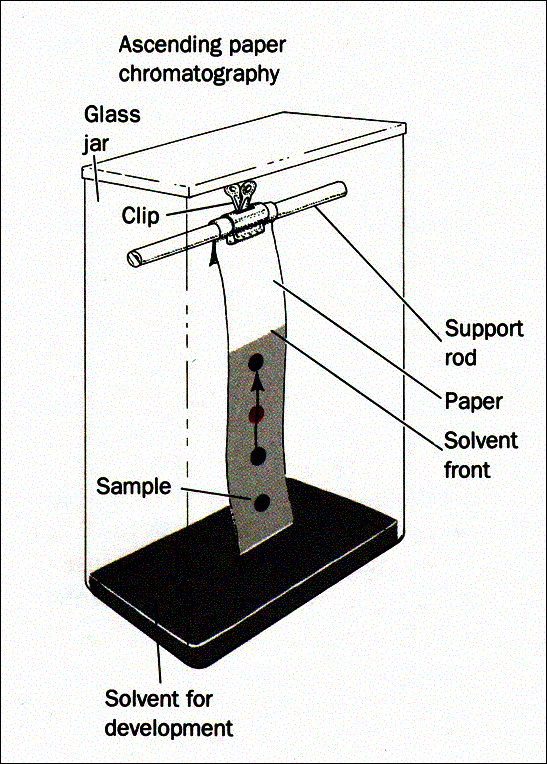
The beaker was set aside undisturbed for about twenty minutes for chromatographic separation to take place. The filter paper was taken out of the beaker when the solvent level got to one inch from the edge of the paper. The level of the solvent was marked using a pencil after which the paper was allowed to dry. Ninhydrin was sprayed all over the paper in a light, uniform layer to enable visualization of the spots, and the paper was dried using a heat lamp. The expanse covered by each spot was measured as well as the distance covered by the solvent. These values were recorded and were used to calculate the Rf values for all the spots.
Results
Table 1: Amino acids and their Rf values
The Rf values showed that unknown amino acid #1 was alanine, whereas unknown amino acid #3 was leucine.
Discussion
Proteins consist of twenty amino acids, which have varying properties in their side chains. Glycine, for example, is the simplest amino acid with a hydrogen atom as the side chain (R-group). Hydrophobic amino acids such as valine and phenylalanine have a hydrophobic R-group that lowers their solubility. Polar amino acids such as glutamate, serine, and lysine have charged groups that increase their solubility depending on the pH of the solution. These properties affected the movement of amino acids on the filter paper.
The nature of the solvent also played a significant role. It was realized that arginine was the most hydrophobic amino acid as it did not show any movement. Unknown #1 had the same color as spot 6, which was alanine. Unknown #3, on the other hand, had the same Rf value as spot 3 (leucine). The filter paper consisted of cellulose-acetate, which yielded positive charges that contributed to the interactions between the samples and the stationary phase. It was important to douse the air in the beaker with fumes from the solvent to minimize the vaporization of the solvent during the chromatographic run.
Conclusion
From this experiment, it was concluded that paper chromatography was a fast and reliable method for the determination of amino acids.
Works Cited
David, A. Grossie, and K. Underwood. Laboratory Guide for Chemistry. 6th ed. 2012. Plymouth, MI: Hayden-McNeil. Print.