Introduction and Foundation of the Research Project
Various types of vaccines have been developed in the United States and Europe to mitigate the effects of the coronavirus disease (COVID-19) pandemic. Among them are Pfizer-BioNTech, Moderna, AstraZeneca/Oxford, and Johnson & Johnson. These vaccines are developed from complex and extensive processes that should ideally take many years to complete. However, the Coronavirus vaccine was developed in less than two years. Hence, it is important to understand the technology and biochemical properties of the vaccine development process that made this happen.
The Emergence of a New Threat
Few clinical problems could become so urgent and relevant to the global agenda that their emergence led to radical changes in all spheres of human life. One of the main threats to the international health system is the recent COVID-19 caused by the pathogenic virus known as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). This disease should be considered new because of the mutational phenomenon, resulting from which the pathogen was able to acquire unique and persistent evolutionary characteristics. In turn, the formation of new coronavirus varieties made it impossible for the existing healthcare systems to respond promptly to the emerging threat due to the lack of experience in dealing with this pathogen. Therefore, the emergence of SARS-CoV-2 was a significant challenge to the global agenda, demonstrating the poor performance of health systems against the emergence of new threats.
It is not only the medical industry that has been severely affected. The severe pressure on health systems has hurt virtually all areas of public life. Unable to cope with the dramatically increased flow of crisis patients, hospitals were overwhelmed and ineffective in helping to deal with the unrelenting pace of the pandemic. In turn, most governments have had to impose severe restrictive measures banning social contacts, public facilities, and significant events (Disantara, 2020). This led to the ruin of small and medium-sized businesses, forcing many commercial organizations to close and disrupt supply chains. Moreover, to contain the rapid rate of infections, many countries hastily closed borders and suspended the issuance of visas, justifying the decision on national security grounds. As a result of the new threat, all spheres have come under pressure, which means that public life, based on the principles of globalism, has been hindered.
Information about the Coronavirus
Coronaviruses are a well-studied group of viral particles containing RNA molecules as genetic material. The name of this family was given to viruses solely because of the similarity of their morphological structure to the solar corona. The lipoprotein envelope of the virus has several spike-like peplomers designed for efficient binding to cell receptors, as shown in Figure 1 below.
Half Sectional View
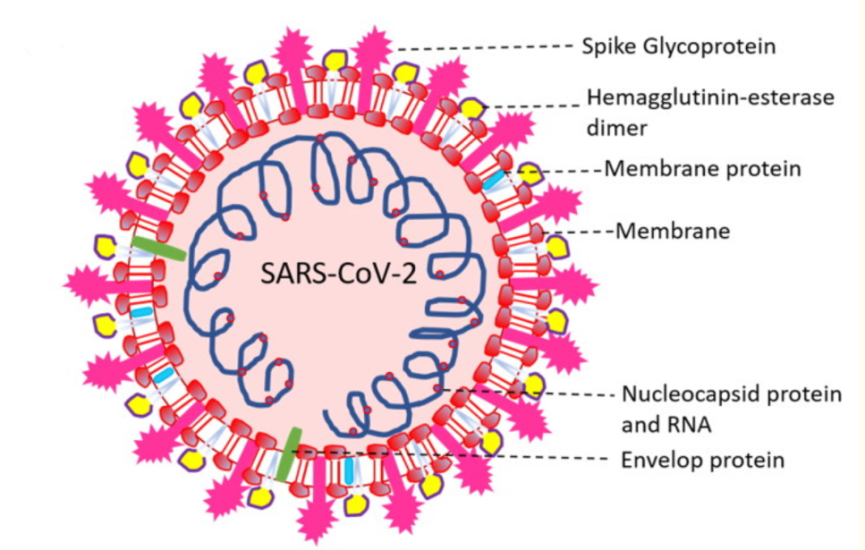
Quarter Sectional View
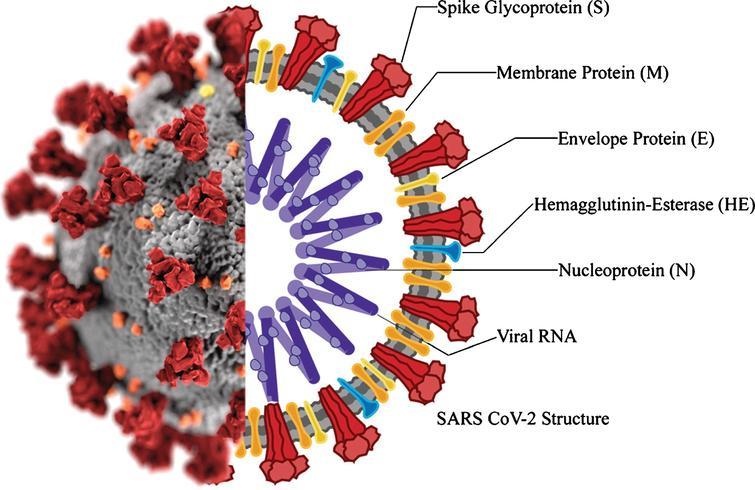
SARS-CoV-2 is entirely consistent with this description and appears to be a naturally occurring virus, although this position still has no clear academic assessment. A central argument in the assumption of a natural origin of the virus causing COVID-19 is the high similarity to SARS-CoV, which caused the SARS epidemic in 2002 (Rokni et al., 2020). On the other hand, genomic studies of the structure of the virus reveal some inaccuracies and assumptions that allow opponents of the natural origin of the pathogen to discuss its laboratory production. For example, the presence of a viral laboratory at the epicenter in Wuhan, China, and the phylogenetic differences from the progenitors, and the multiplicity of mutated forms provide the basis for skepticism. At the same time, the current virus has a steady tendency to evolve through mutagenic processes of genetic material. This creates a wide variety of similar pathogens with various functionalities and makes it much more challenging to fight them effectively.
Morphological Characteristics
The morphological and genomic characteristics of the virus have already been sufficiently studied. According to Varga et al. (2020), the virions are roughly spherical or oval, with an effective diameter of about 180 nanometers. Morphological analysis of the pathogen shows the presence of multiple lipoprotein outgrowths of the viral envelope that fulfill the functional role of binding to target cell receptors and starting mechanisms to trigger infection. Thus, each peplomer consists of a protein S complex represented by three proteins. S1 at the top of the spike interacts with the target cell receptors, while S2 and S2‘ carry out a biochemical fusion of the viral envelope with the host cell membrane to facilitate the injection of genomic material (Varga et al., 2020). Figure 2 below shows different color codes for the varied types of proteins characterizing the virus and how they bind in the human body to cause infections.
Notably, each peplomer is generally mobile and is a hinge mechanism, which means that the attachment of SARS-CoV-2 to the cell surface proves to be more efficient for the virus. In addition to these proteins, the viral particle also has biopolymers that form the nucleocapsid envelope as shown in Figure 1 above. Such proteins package the viral RNA and play a fundamental role in the assembly of the viral particle, but the structure of the coronavirus nucleocapsid is not currently described in any detail (Covián et al., 2020). From the known information, it should be noted that the internal proteins of the capsid are bound in a chain no thicker than 15 nanometers, with the capsid as a whole not occupying the entire free volume of the pathogenic particle. Inside the nucleocapsid, as is customary for viruses, there is genomic material.
Genomic Characteristics
SARS-CoV-2 is a typical RNA-containing coronavirus with reliable morphological evidence of belonging to this taxon. The virus contains a single-stranded RNA measuring approximately 29.8 base pairs (Rokni et al., 2020). The genome itself has six open reading frames, which are characteristic of all coronaviruses and genes unique to SARS-CoV-2 responsible for synthesizing matrix and nucleocapsid proteins. SARS-CoV-2 should be assigned to the beta-coronaviruses (Coronaviridae family) among which there is SARS-CoV, which caused SARS pneumonia. This allows the current pathogen to be evaluated as closely related to preexisting forms but mutated and has crossed the interspecies barrier.
Aetiological Evidence
SARS-CoV-2 is a pathogen of the respiratory system of humans. The virus is capable of causing acute respiratory viral infections, including respiratory failure (Xia et al., 2021). Figure 3 below is a sample of a functional and infected lung.
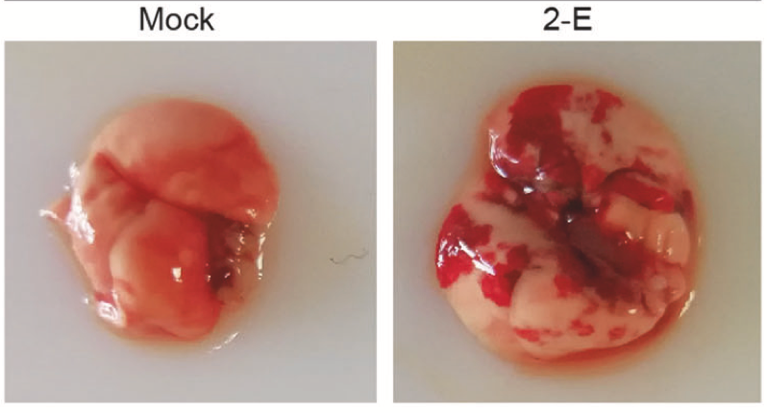
The critical entry gates for pathogen entry are the epithelium of the upper respiratory tract. Consequently, the mode of transmission of the virus involves inhaling air contaminated with viral particles, especially near the source of infection, namely a sick person or hospital. SARS-CoV-2 first attaches to target cells that have ACE2 receptors, including in the cells of the nasopharynx. Figure 4 below shows how the pathogen penegtrates through the hemolytic barrier of the lung to cause infections.
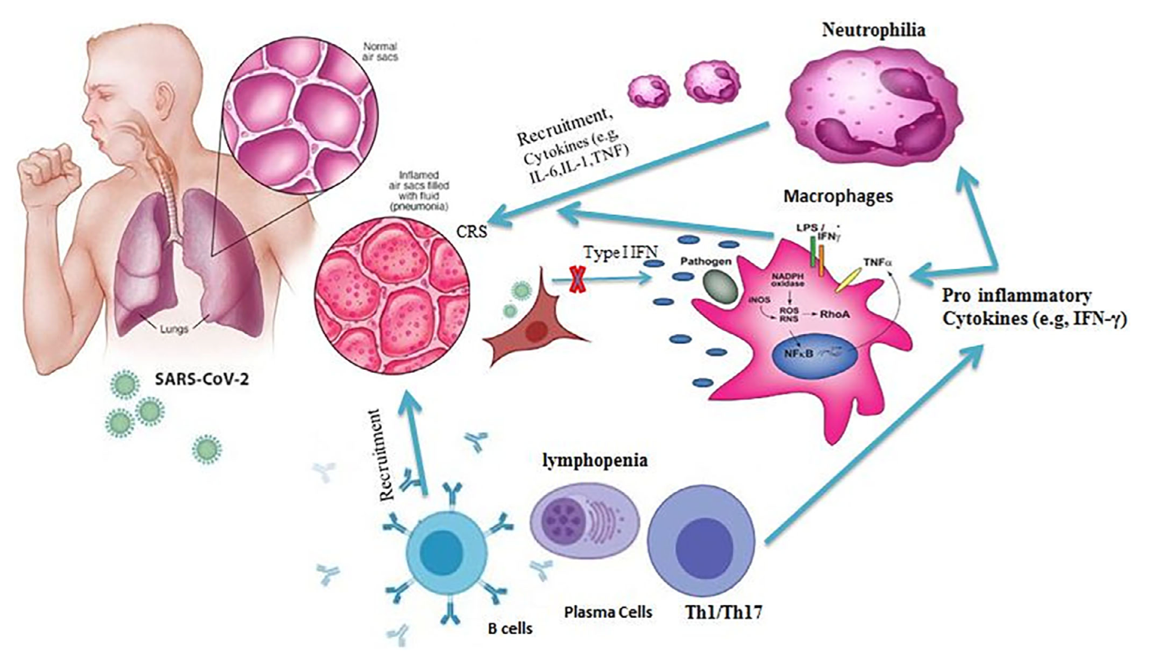
Infection could result in a change in a patient’s sense of smell at an early stage of the disease and may be indicative of nasopharyngeal mucosal edema.
Types of COVID-19 Variants
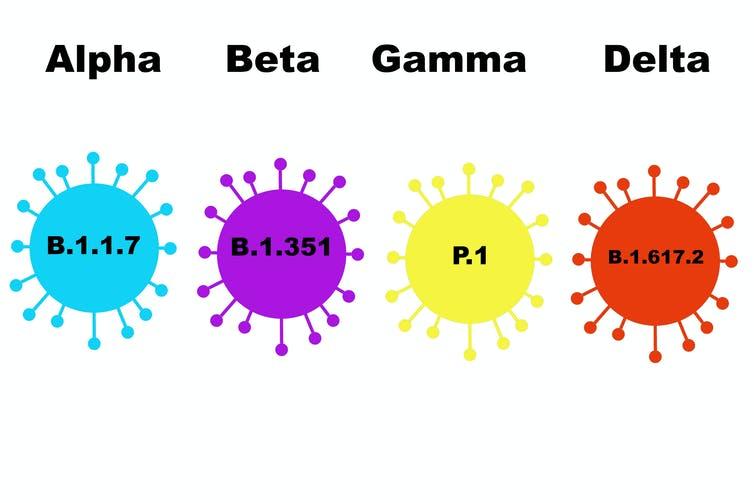
The COVID-19 pandemic is one of the world’s most significant public health issues to have affected the human population in the last 100 years. It has taken a long time to be contained because it is continuously evolving. Scientists have identified different variants relating to its occurrence, including Alpha, Beta, Gamma, Delta, and Omicron (World Health Organization, 2021). The Gamma variant was first detected in Japan and Brazil, while the Beta variant was first detected in South Africa (CDC, 2021). Comparatively, the Alpha variant was detected in the United Kingdom and the Omicron variant has been reported in multiple locations around the world (World Health Organization, 2021). In the context of this discussion, the focus will be on the Delta and Omicron variants which are the most deadly, transmissible and yet to be fully understood. Each of these segments has one or more mutations that differentiate it from one another but all come from one common source or root. The World Health Organization (2021) introduced a naming system for these different variants according to figure 5 below
The Delta variant was first detected in India and it features a triple mutation of the virus. These mutations are classified according to three codes. The first two include E484Q, which helps the virus to evade antibody detection and L452R, which allows it to replicate more frequently and latch on to the human cell more tightly (CDC, 2021). The last mutation is the P681R, which makes the virus more infectious. The net effect of the Delta variant compared to other existing mutations is that it is more transmissible compared to its original version (World Health Organization, 2021). Some report suggests that it could be 40% and 60% more transmissible compared to other types of variants (Kindrachuk & Shaw, 2021). However, it is still unclear whether people who contract this type of virus become sicker or not.
The Omicron variant is the latest mutation of the COVID-19 virus and unlike other variants that were reported in one or more selected countries, it has been in multiple nations. According to figure 6 below, this variant is registered by the code B.1.1.529
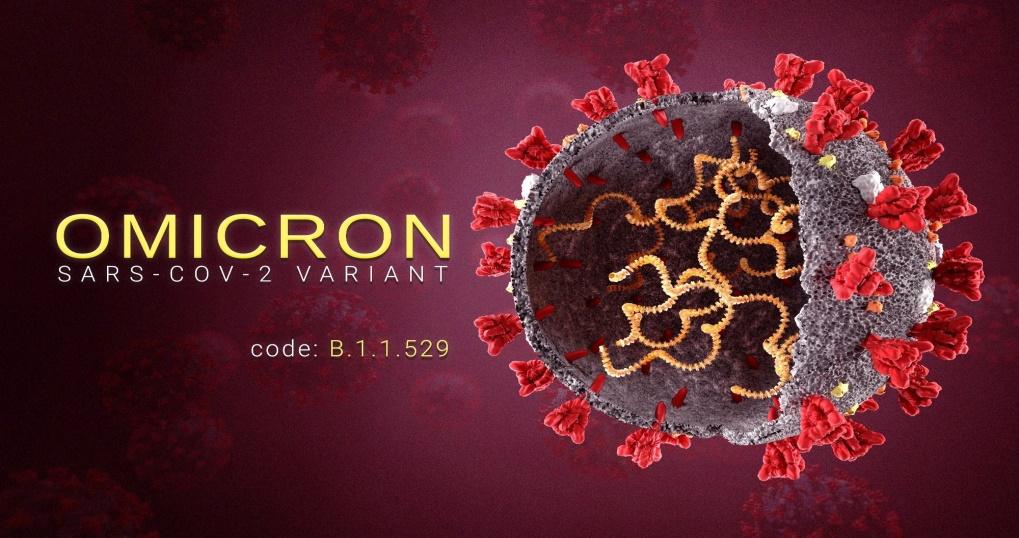
The CDC (2021), in collaboration with the World Health Organization (2021) and other agencies are still learning about the Omicron variant, especially about the effects of vaccines on its spread. However, preliminary research suggests that it is more transmissible compared to the poroginmal SARS-CoV-2 (Bose, 2021). There are concerns that this variant may affect people who have been fully vaccinated (Bose, 2021). This concern means that there may be limited efficacy of existing vaccines against this variant.
Vaccination as a Solution to the COVID-19 Problem
Every time humanity is confronted with a new infectious disease, the efforts of clinical laboratories rush to find an effective cure, diagnostic tests, and a vaccine. A vaccine to stimulate the formation of artificial immunity is an excellent solution, proven over time and by many generations of physicians. However, the most effective vaccine must be obtained using modern instrumental biotechnology methods and provide a targeted response to a particular virus strain. To achieve these goals, technologies have previously been developed and approved by the European and international responsible authorities for obtaining vaccine material: from attenuated, non-pathogenic pathogens to recombinant vectors carrying genetically modified copies of the virus antigens. This study discusses the most popular forms of vaccine that have gained public acceptance worldwide: Pfizer-BioNTech, Moderna, AstraZeneca/Oxford, and Johnson & Johnson.
Research Gap
There have been many commentaries made in the popular press about the development of the COVID-19 vaccines with much of the attention drawn towards making it available to all countries. However, comparably little has been published in the popular press conecerning the the biochemical and technological advances used to develop these vaccines or how they help the human body develop immunity to the virus. Stemming from this background, the proposed study will focus on understanding the biochemistry behind the development of COVID-19 vaccines and their efficacy in performing their core function, which is to help the human body develop immunity. Therefore, the proposed study will link two elements of analysis in the investigation: the biochemistry of the vaccines and their function in helping the body develop immunity against COVID-19 spike proteins.
Problem Statement
The emergence of the threat of SARS-CoV-2, which causes the dangerous respiratory tract infection COVID-19, has served as a significant challenge for the world. To develop a collective immunity and thus protect humanity, many laboratories worldwide have developed vaccine formulations with the potential to fight COVID-19. The vaccines (Pfizer-BioNTech, Moderna, AstraZeneca/Oxford, and Johnson & Johnson) have qualitatively different ways of biochemically protecting the body and different efficacy.
Research Aim
The aim of this research project is to understand the biochemical and technological foundations in the development of different COVID-19 vaccines both in USA and Europe (Pfizer-BioNTech, Moderna, AstraZeneca/Oxford, and Johnson & Johnson). The purpose of this study is to conduct a detailed review of the available literature revealing the biochemical and technological features of the clinical effects of the four vaccines discussed.
Research Objectives
- Identify the two types of COVID-19 vaccines and how do they work.
- Assess how the biochemical compositions of the DNA and RNA protein in the development of the Coronavirus vaccine.
- Explore the structure, genome, and mode of infection for SARS-COV-2.
- Discuss the scientific basis behind the various vaccine technologies.
- Investigate the role of mRNA vaccine technology in the development of the Coronavirus vaccines and how they prevent COVID-19.
- Examine the relative effectiveness of the four vaccines in reducing infections resulting from exposure to the various virus variants circulating in the USA and Europe.
- Assess how the biochemical composition of the coronavirus vaccine guarantees its safety in humans.
Scope and Limitations
This study was performed as part of a master’s degree program and and is based on a literature search to address the research objectives. Some limitations that may hinder the ablity to scale the findings concerning the relative effectiveness of the various vaccines should be emphasized. First, vaccine preparations are still undergoing clinical trials on a large scale, so it is too early to assert definitive credible data on their safety and efficacy. Second, the sources selected for this paper were initially filtered for English language. There remains the possibility of ignoring potentially valuable data from those publications that have not yet been translated. Finally, only four current vaccines were studied in the paper, which means that the results obtained can hardly be approximated for all the preventive vaccines.
Significance of the Study
Few scientific papers to date have done a comprehensive review of several vaccines simultaneously, thus providing a comparative analysis. On the other hand, the study is characterized by high practical relevance, as the results shed light on the efficacy and safety parameters of the vaccines studied. Moreover, the source review reveals useful information about the biochemical composition of the activity of such vaccines, and therefore the research work is meaningful for students, physicians, and other interested parties. The findings of this study are important to adult learners who have an interest in understanding the relationship between biochemistry and vaccine technology.
Literature Review
Among the existing clinical tools for controlling infectious diseases, vaccines should be given special attention. In contrast to classical drug treatment, vaccination is a preventive therapy to generate an immune response (Covián et al., 2020). The general pattern of forming an immune response through vaccines is the same. By injecting a foreign antigen into the patient’s body, it produces its antibodies, making it easier to recognize and capture the pathogen if future infections occur. Each vaccine has a targeted effect against a specific infectious agent since the mechanism of action of the vaccine aims to create antibodies to specific antigens. Vaccines differ in their methodological approaches; either whole viruses or their fragments can be used as the critical protective agent. This literature review examines various aspects of the vaccination phenomenon. The review focuses on the specific vaccine products with the greatest international acceptance as a means of controlling COVID-19 infection, namely Pfizer-BioNTech, Moderna, AstraZeneca/Oxford, and Johnson & Johnson.
Types of COVID-19 Vaccines
Vaccines were thought to be defined as introducing only a dead or weakened pathogen into the human body so that the immune system could develop a preventive response to the active infectious agent. In reality, this information is outdated, as the methodology of modern vaccines has evolved considerably (Woodruff et al., 2020). Current biotechnological advances have made it possible to produce vaccines using two uniquely different technologies. The first one is Messenger RNA (mRNA) and the second is adenovirus-based vaccines. Both techniques use different methods for developing vaccines with mRNA relying on protein gene modifications and the latter being focused on DNA genome recalibration. AstraZeneca/Oxford and Johnson & Johnson have been developed using the latter technology, while Pfizer-BioNTech and Moderna have been developed using the former (Venkadapathi et al., 2021). Overall, the action of vaccines is based on the interaction of the genetic material of the injected drug with the internal structures of the immune cells.
Biochemical Basis Of The Two Different Types Of Vaccines
mRNA-Based Vaccines
Messenger RNA (mRNA) is one type of technology used to develop COVID-19 vaccines. Pfizer and Modena are the two major brands of vaccines using this technology. It works by modifying protein genes in cells that are relevant in fighting infections by deleting aspects of their molecular formation that are unable to detect disease spread and adding new properties that allow the body to develop antibodies to fight infections (Tenchov et al., 2021). Therefore, synthetic mRNA is responsible for protein modifications in the body with the view of strengthening the body’s immune system. Given that the mRNA technology is relevant to protein production, having the ability to modify the process means that it is possible to increase a cell’s ability to resist virus infections (Garde, 2020). In theory, it means that it is possible to tell the cells which antibodies to make to vaccinate themselves against infections and the enzymes to create to reverse disease progression.
COVID-19 vaccines that that have been developed with the mRNA technology have high efficacy levels. Both Moderna and Pfeizer vaccines, which were developed with this technology in mind, have N1-methyl-pseudouridine-modified mRNA with efficacy levels above 90% (Xie, 2021). This mRNA technology has been used to modify SARS-COVID-19 Spike protein.From this background, scientists have used mRNA technology to develop two mutations for the SARS-CoV-2 spike protein, which are K986P and V987P (Garde, 2020). They have been effective in providing additional data relating to the viruses’s nature.
The mRNA technology has not only been used in the development of COVID vaccines because researchers have for a long time used it to modify cell structure and direct it to perform functions that were hitherto unfathomable. However, in the development of the COVID-19 vaccines, it was established that the modified spiked protein triggered an immune response from the body, thereby making it difficult to alter molecular structures for antibody development (Bose, 2021). Consequently, researchers were forced to alter aspects of the cell to bypass this immune trigger (Garde, 2020). The result would be that the modified enzymes would bypass the immune system and develop defences against the virus.
Once complete, the RNA is absorbed into the body through muscles and it is later absorbed into the immune system to develop immunity against COVID-19. This was made possible by a trans-membrane anchor visible on the top layer of the cell, which made it possible for the immune system to recognize its presence (Bose, 2021). Consequently, antibodies and T-cells were developed and nurtured to provide a natural resistance to the virus. Some trials established that mRNA technology could simply be injected in a tissue using vitro transcription of mRNA and the desired efficacy levels would be attained (Garde, 2020). The nucleic acid typically enters cells through adsorption of the lipid nanoparticles. Endocytosis is achieved in the process but penetration occurs on the cell membrane.
The cell membranes typically consist of a negative charge but the lipid nanoparticles have a positive one. The opposite charge is responsible for membrane fusion and endocytosis. Nucleic acids would have entered the cell at this point and it is paramount for them to be released in the cell membrane to allow for nucleic acid delivery. The release of nucleic acids is likely to occur when the anionic liquids in the cells are released. The use of mRNA technology in vaccine development was responsible for the relatively fast-paced nature of developing COVID-19 vaccines because it only took a couple of months to complete the research, thereby leaving room for vaccine development.
Stabilizing the Spike Protein
Innate immunogenicity is another challenge that could occur due to the use of mRNA vaccines, thereby causing its ineffectiveness. It refers to the body’s response to the introduction of a foreign protein in the body because it will naturally try to fight it off (Nance & Meier, 2021). This problem causes a destabilization of the spike protein, which should ideally be used to help the body develop antibodies to detect and fight the COVID-19 virus. The solution to this challenge was found in the stabilization of mRNA using pseudouridine (Nance & Meier, 2021). Both Pfizer-BioNTech and Moderna vaccines have this stabilization mechanism and are consequently reliable because of its use.
The use of mRNA technology to develop COVID-19 vaccines is responsible for some of the early breakthroughs in the development of vaccines. However, the possibility of nucleus degradation of vitro transcribed mRNA due to transportation challenges made it difficult to avail the vaccines in different locations or countries around the world. The vaccines also lacked nucleobase N1-methylpseudouridine, which should ideally help the vaccine to overcome the body’s immune triggers and promote protein production (Nance & Meier, 2021). Based on the above insights, there was need to develop new technology that would slow down the process the process of RNA degradation until the vaccine reached the intended audience.
Lipid nanoparticles (LNPs) were introduced to aid in delivery and based on its success in doing so, they have been hailed as one of the success factors for the production and use of vaccines using mRNA technology (Nance & Meier, 2021). The structure of the lipid nanoparticles appears in figure 7 below.
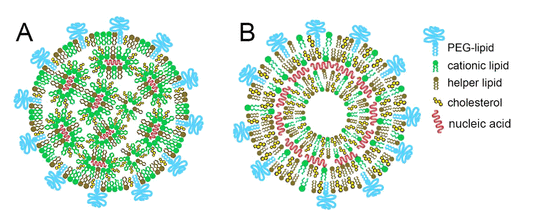
The first illustration (A) shows the nucleic acid organized in reverse, where the lipid micelles are inside the nanoparticles. In the second illustration (B), the nucleic acids are intercalated between lipid bilayers. Figure 8 below shows the structural features of uridine and the supportive materials.
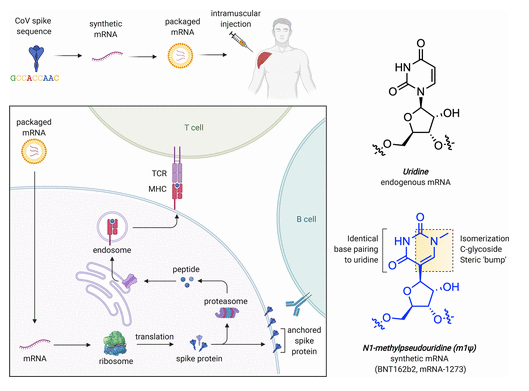
According to figure 8 above, the COVID-19 spike sequence is modified using synthetic mRNA, which is then packaged and transmitted into the body using injections on the arm. The chemical composition of vaccines developed using the mRNA technologies are fundamentally different from others in the market because they include water, salt, sugar and fat (Nance & Meier, 2021). The use of the mRNA technology has led to the development of different sequencing techniques for Pfizer-BioNTech and Moderna vaccines. Figure 9 below shows the sequencing format for Pfizer-BioNTech, as an example.
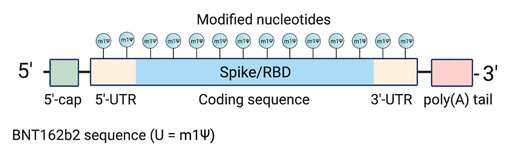
The above diagram shows that the Pfeizer vaccine has a linear sequence with an active payload of 4284 nucleotide (Cross, 2020). This sequence has five key elements that serve various functions. They include an active compound known as “Cap 1,” which helps to recruit Ribosome and prevents the RnA from degrading, an optimized Kozak sequence that helps in translation functions, codon-optimized coding sequence for the production of SARS-CoV-2 spike glycoprotein, 3′-UTR for stabilizing the RNA, and a poly(adenosine) tracts for increasing RNA stability (Nance & Meier, 2021). These features show that the mRNA vaccine was effectively designed but skeptics believe that the process was rushed (Tenchov et al., 2021). They are wrong because the mRNA research that underpins the development of the vaccine is a product of decades of research in the field of RNA biology.
Cellular Uptake
mRNA requires the safe and effective delivery of vaccines using lipid nanoparticles. This compound not only protects the nucleus form degradation but also aids in the cellular uptake of the virus (Tenchov et al., 2021). Functioning in VIVO requires the lipid nanoparticles to overcome several cell barriers that should protect it from nucleus degradation and evade interception by the body’s immune system. At the same time, the lipid nanoparticles should escape endosomes to reach the cytoplasm where data encoding will occur. Figure 10 below highlights delivery barriers that may impede the effective utilization of this technology in vaccine development
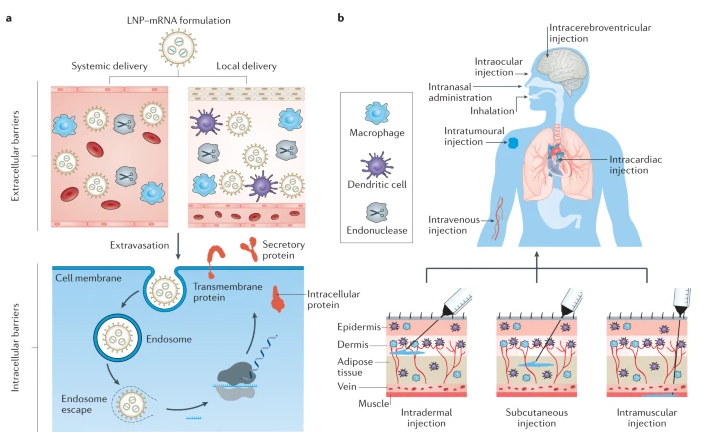
Based on the above-mentioned figure, the main types of barriers affecting lipid nanoparticles are intracellular and extracellular. Extracellular barriers emerge in two segments – systemic delivery and local delivery, while intracellular barriers are concerned with extravasation and endosome escapes. The lipid nanoparticles also stabilize spike proteins based on the molecular structure of the compounds. This feature is found in positively charged lipids, which provide stability to nucleic acids (Tenchov et al., 2021). This feature makes them resistant to nucleic degradation, thereby making it possible to prevent nucleic degradation.
Adenovirus-Based Vaccines
Adenovirus is another technology used to develop COVID-19 vaccines. It was considered as an appropriate platform for vaccine development because of its potency and ability to initiate a balanced immune response (Tenchov et al., 2021). The technology works by DNA genome recalibration to make it more effective in developing antibodies that would fight off the COVID 19 virus. As depicted in figure 11 below, the DNA genome to be modified is confined within the cell structure highlighted below, characterized by fibers, a Hexon structure and a pentagon base. The structure of the cell is as depicted below and it contains an outer protein layer, which safeguards an inner one that contains a nuclear protein core.
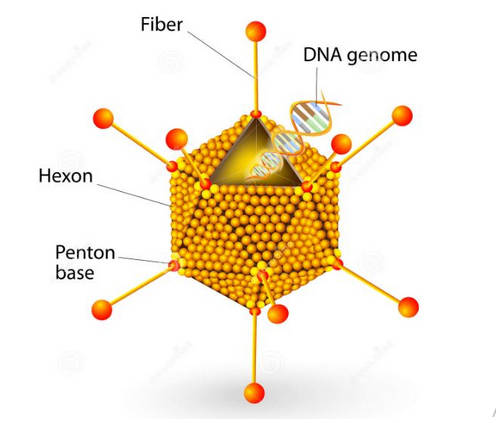
It is possible to change the DNA genome structure of the above cell despite it being thick and containing measurements of 34~43 kb (Creative Biolabs, 2021). Viral DNA replication occurs in the early transcription units and they are primarily responsible for by passing a host’s immunity defenses, thereby avoinding the problem that mRNA vaccines have had in triggering a host’s immunity defenses due to the addition of foreign proteins. Therefore, late transmission units associated with adenovirus production encodes the viral structural elements of the cells to create a new molecular structure that would be essential in developing antibodies for fighting the COVID-19 virus. Thus, this type of technology is premised on changing the structure of DNA genomes to boost one’s immunity based on research that has allowed biologists to understand its basic structure.
Manipulating the structure of the DNA genome has made it possible to develop effective medicine through adenovirus-based vaccines. Based on this principle, adenoviruses have a double-stranded DNA genome that is well understood and can be manipulated to realize improved objectivity of vaccine development (Creative Biolabs, 2021). Due to their immunogenicity, adenoviruses have been highlighted as one of the critical bases for the development of COVID-19 vaccines. Their ability to induce host protection in various animals has also increased hopes in medical research that its use in humans can be equally as effective.
Two strategies have been used to design adenoviral vectors. The first one involves homologous recombination of two components with the first one being a combination of adenoviral genome and shuttle plasmid. The latter is transgene cassette, which is associated with the typical mammalian system (Creative Biolabs, 2021). Figure 12 below shows how the homologous combination occurs in mammalian systems.
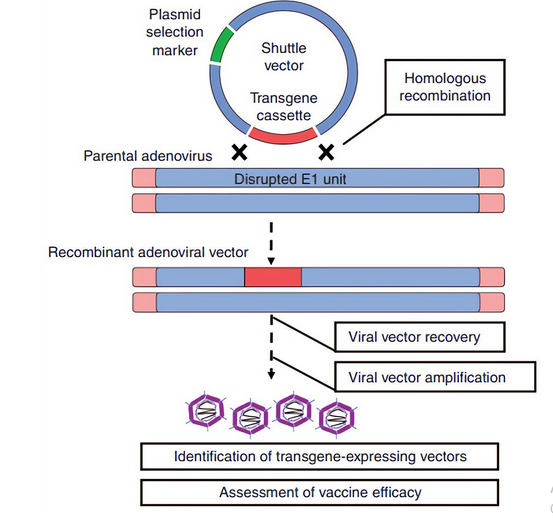
Figure 12 above shows how adenoviral-vectored vaccines are developed through homologous combination. The second technique of vaccine development does not necessarily require homologous combination because it involves a cloning method where the transgene cassette is directly integrated into the adenoviral backbone. Figure 13 below depicts how this strategy works because it depends on direct cloning activities through the injection of adenoviral vector, thereby bypassing the need for homologous recombination as highlighted above.
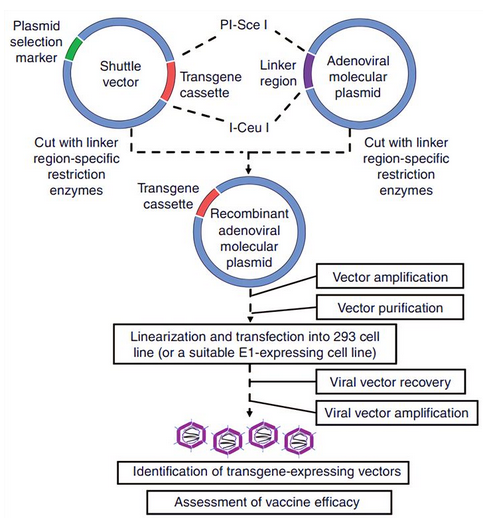
To promote the use of adenoviruses in humans and in the development of vaccines, genetic engineering has been proposed as one of the most effective ways of doing so (Creative Biolabs, 2021). The third generation adenovirus vectors are realized when deleting almost all generic sequencing protocols except those that involve packaging processes. The adenovectors enter the immune system by reviewing how adenoviruses combine with different receptors.
Adenovirus-based vaccines have not only been used to develop COVID-19 vaccines but also those of Ebola. This means that there is a history of its use beyond the current pandemic. Its use in developing COVID-19 vaccines is still dependent on the findings of large clinical trials that are currently being undertaken but there is promise that the use of recombinant adenovector platforms will yield positive results in the end (Chang, 2021). These vaccines are being developed using either of the three platforms highlighted, which are Had5, Had26, or of ChAdOx-1 (Chang, 2021). Based on this technology, Adenoviruses have a strong potential of creating strong cellular and temporal immunity. Adenoviruses also have a natural tropism, which allows them to use respiratory mucosa to develop effective immune responses. In this regard, Adenovirus vaccines differ from all others in the market, especially in the treatment of the COVID-19 pandemic because they play the role of delivery shuttles (Hackethal, 2021). They rely on adenovirus, which has typically been reengineered to prevent multiplication or replication of DNA genomes, hence offering protection from viruses.
Overall, unique molecular characteristics of Adenovirus vaccines have made it a candidate for consideration in the development of the COVID-19 vaccines. The efficacy of the technology used to develop this type of vaccines lies in its ability to delete certain genes or attribute to allow for the development of properties that would aid in disease prevention. Particularly, this technology makes it possible to delete E1 and E3 viral genes that make it possible for the Corona virus to replicate quickly (Servick, 2020). Upon deletion, these components are replaced with a transgene of interest that would prevent its replication. This action prevents the virus from reproducing its genomic sequence after delivery, thereby making it possible to control its spread.
Overall, a comparison of the two types of technologies used to develop COVID-19 vaccines shows that each one of them has its merits and demerits. However, Adenoviral vector vaccines appear to be a significantly more powerful platform for developing vaccines compared to mRNA. This is because they attract lower costs and a higher themostability index (Mendonça et al., 2021). However, the two technologies used in developing these vaccines remain a core part of medical research studies, especially as they relate to the development of vaccines beyond the scope of the pandemic.
Efficacy of the COVID-19 Vaccines
Most approved vaccines are still being clinically evaluated on a large sample of people, gathering information about possible side effects and adverse events. For this reason, the effectiveness of the vaccines is estimated to be relative, not finite. Pfizer-BioNTech was initially rated at 95% efficacy in suppressing symptomatic disease when approved by the FDA, with efficacy remaining at 90% when fully immunized (Thompson et al., 2021). Pfizer-BioNTech’s efficacy was only 88% against new strains of SARS-CoV-2. The proven efficacy of Moderna is slightly lower at 94.1%, with efficacy decreasing to 90% for complete immunization. Johnson & Johnson’s estimated efficacy is only 72% and 86% for severe disease (Zimmer et al., 2021). Finally, the overall efficacy for the AstraZeneca/Oxford vector vaccine is less than 76% and 100% for therapy of complicated cases (Vogel & Kupferschmidt, 2021). In general, without a plausibility analysis, it can be seen that mRNA vaccines appear to be more effective compared to adenoviral vaccines, which have, among others, cases of severe side effects.
Vector vaccines use weak viral agents that transfer the gene for the antigen into human cells where it is reproduced by the cells genetic machinery. mRNA vaccines contain a modified version of the mRNA for the antigen surrounded by a lipid shell. The proven efficacy of mRNA vaccines is comparably higher, with less overall evidence of possible adverse events. Thus, vaccines such as Pfizer-BioNTech and Moderna show better performance compared to Johnson & Johnson’s and AstraZeneca/Oxford.
Research Methodology
In order to provide a comprehensive survey of the comparative effectiveness of the vaccines and the virus itself, a systematic review of COVID-19 related journals is required. The proposed study is based on a review of biochemistry and medicinal chemistry journals that have discussed the research topic. I thoroughly examined multiple network databases with exact information about different issues present in the paper. Also, open access sources were included to demonstrate the non-for-profit character of the medical research on the topic. Because the COVID-19 epidemic situation and the vaccines’ effectiveness are of crucial meaning for nowadays world, there is a plentiful amount of surveys with free access. Thus, I decided to investigate and evaluate the information from various online peer-reviewed journals.
Study Design
The research objectives were clearly defined, and I limited the scope of the research to present specific information about the coronavirus and vaccines preventing its spread and negative causes for the population. Information from various journals of the USA and Europe were included in this project. The reason behind this choice is that the selected data sources could be universally accessed and adequately understood by the majority of people. The sources for the survey comprise databases with scientific articles from various medical journals, scientific publications from professionals in the topic, which are reviewed in detail. The journals were evaluated on the subject of credibility and significance for the research to supply only valuable and reliable data. Finally, I compared the information from the selected sources and deliberately picked the most appropriate, relevant and presentable material for inclusion. Thus, the research contains the processed information stated in a concise manner. The following journal sources were explored.
Multidisciplinary Digital Publishing Institute (MDPI)
MDPI is a publisher of open access scientific journals. The first valuable source with possibilities for searching for information concerning the current state of scientific knowledge about the coronavirus varieties and recently developed vaccines is MDPI. The organization is primarily concerned with publishing scientific articles in its journals focused on various areas of medicine and biology. On the MDPI website, numerous researches from academic communities are published on the open access policy. I used the PubMed database, a part of the MDPI global project, since it contains high-quality papers in an online access form. I examined several studies related to the topic of the survey and decided to introduce the most important findings.
Frontiers
The other journals with ample medicine and biological content could be found on Frontiers. The platform presents open access works from various peer-reviewed scientific journals predominantly in English. Yet, authors from different countries participate in the process of global information exchange, which makes the database even more significant because of its multicultural perspective. We searched information specifically in the subsections of immunology, molecular biology, and biochemistry for information related to virus infections and their prevention. The publisher provides a peer-review system for each work present in their service so that data/information is guaranteed to be credible.
National Institute of Health (NIH)
National Institute of Health is apparently essential for the search procedure during the research about a disease. The database links multiple websites with relevant medical literature, as well as journals of the USA. I decided to use the NIH database because of its vast online archives with reviews of researches or even full-text articles on the topic. NIH aims at the global exchange of information to enhance the health and well-being of the US population and the whole world. Such values yield to the prosperity of novel data on the currently important issues. Thus, I considered it a valuable resource and examined the various content that it supplies.
Centers for Disease Control and Prevention (CDC)
The great chance of lethal outcome caused by COVID-19 disease is incredibly relatable to the CDC’s mission. The organization is remarkable for its involvement in the publication of medications-specific researches. The quantitative approach is significant for any comprehensive research on a topic that needs precise information. Since COVID-19 vaccines belong to such a topic, the CDC database was helpful for the research.
Nature
Nature is an international journal with a well-grounded reputation, and it provides access to numerous researches and articles of different complexity levels. Moreover, Nature Partner Journals, a project under Nature supervision, publishes articles in the subsection “Vaccines,” which is of particular importance for the research. All the materials distributed by the organization are publicly available in the online regime. Most papers contain suitable statistics and illustrative graphs, a significant feature for the content related to medicine and science behind it. Therefore, entailing the information from Nature was apparently necessary for this study.
New England Journal of Medicine (NEJM)
The New England Journal of Medicine, although not an international organization, yet is essential for the search for medical information. Its main goal is to provide an opportunity for professionals in the sphere of diseases prevention and treatment to share their manuscripts. The administration of the journal examines all the works and delivers detailed reviews of their value and credibility. The advanced scientific materials stored on the organization’s website are related explicitly to the current state of global health. I searched through the database to attain the most practical information about coronavirus and the various types of vaccines.
World Health Organization (WHO)
The World Health Organization is undoubtedly a reputable source of information about the health state of people from various countries and groups of the population. The impact of virus infection is announced on the WHO website and updated frequently. Additionally, WHO links the statistical information about the disease’s influence on humans with scientific summaries. Also, the organization is responsible for evaluating the effectiveness of the currently available vaccines and medications. Hence, WHO is considered necessary to this research.
Journal Science
The Journal Science also contains a series a useful materials that are frelevant to our understanding of vaccines. It hosts a series of published medical journals on vaccine development and highlights the progress made in scientific research on several key areas of biomedical research. The materials contained in this database will be useful in understanding the background research that informs the technologies used in vaccine development. Information relating to future directions of research will also be unearthed from materials obtained through this journal database.
Analysis and Findings
For the purpose of conducting meaningful and accurate research, multiple reading sources available in scientific journals were examined. For this research, I analyzed the research of reputable authors related to the various type of COVID-19 vaccines. The four vaccines described in the study are used predominantly in Europe and USA. It is logical to evaluate their effectiveness in the areas of their most frequent application. An analysis of of the types of the COVID-19 vaccines is as follows.
Types of COVID-19 Vaccines: mRNA Vaccine and Viral Vector Vaccine
A coronavirus disease 2019 (COVID-19) vaccine can prevent people from getting COVID-19 or from becoming seriously ill or dying due to COVID-19. Each COVID-19 vaccine causes the immune system to create antibodies to fight COVID-19. COVID-19 vaccines make use of a spikelike structure on the surface of the COVID-19 virus called S protein. The S protein helps the COVID-19 virus get inside the cells and start an infection. The two main types of COVID-19 vaccines are mRNA Vaccine and Viral Vector Vaccine.
Messenger RNA (mRNA) Vaccine
This type of vaccine uses genetically engineered mRNA to give the cells instructions for how to make the S protein found on the surface of the COVID-19 virus. After vaccination, the immune cells start making the S protein pieces and displaying them on cell surfaces. This causes the body to create antibodies, and if a person become infected with the COVID-19 virus, these antibodies will fight the virus. After delivering instructions, the mRNA is immediately broken down. It never enters the nucleus of the cells, where the DNA is kept. Both the Pfizer-BioNTech and the Moderna COVID-19 vaccines use mRNA. The mechanism for mRNA development is highlighted in Figure 14 below.
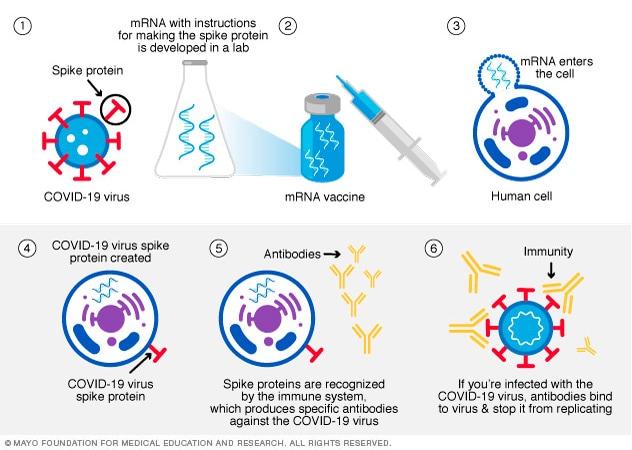
Viral Vector Vaccine
In this type of vaccine, genetic material from the COVID-19 virus is placed in a modified version of a different virus (viral vector). When the viral vector gets into the cells, it delivers genetic material from the COVID-19 virus that gives the cells instructions to make copies of the S protein. Once the cells display the S proteins on their surfaces, the immune system responds by creating antibodies and defensive white blood cells. If someone later become infected with the COVID-19 virus, the antibodies will fight the virus. Viral vector vaccines cannot cause you to become infected with the COVID-19 virus or the viral vector virus. Also, the genetic material that’s delivered doesn’t become part of your DNA. Johnson & Johnson and AstraZeneca/Oxford are viral vector vaccines. The viral vector vaccine mechanism is highlighted in figure 15 below.
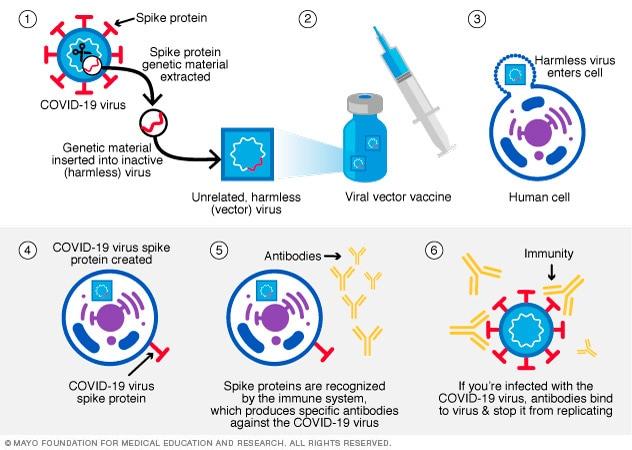
The Use of Online Databases and Information Portals
The two types of COVID-19 vaccines were searched through the majority of the discussed databases. The journals provided highly detailed information about the work of the vaccines of mRNA and Vector type. As such, Inagaki et al. (2021), who published their paper in MDPI, demonstrated a sophisticated description of the Vector vaccines’ work. Covián et al. (2020) researched the mechanisms of the vaccines’ work and outlined them in their paper in Frontiers of Immunology. Furthermore, the introductory information of Thompson et al. (2021) read from CDC presented an updated view on the modern two types of vaccines and their specific structure for preventing COVID-19 disease. Finally, Pancevski (2021), in his article for the Wall Street Journal, briefly describes the difference between the practical mechanisms of AstraZeneca/Oxford and Johnson & Johnson vaccines. Thus, the review of information on the question of the different vaccines’ work is thoroughly discussed in the scientific community and popular media.
Next, I aimed to describe the scientific basis between the technologies that provide the effectiveness of the vaccines serving for preventing the spread of coronaviruses. Several pieces of research were used to deliver information about the mechanisms of the vaccines. Thus, NIH, CDC, and Frontiers (specifically Immunology subsection) reveal the most appropriate methods for preventing the infection spread, which could be expected from the specificity of topics that the databases aim to cover. Additionally, Lainscek (2020) published a paper in MDPI where meaningful pieces of information for the vaccines are demonstrated illustratively.
The biochemical composition of the RNA protein is also considered significant for the comprehension of the vaccines’ work, their development, evaluation, and improvement. The structure of the nuclear parts was studied in great detail in the early stages of biochemistry research; however, the study of its practical application in the sphere of immunology has been considered valuable only recently. The researchers provided an exemplary overview of the technologies based on mRNA. This information helps to gain an understanding of the prevention mechanisms of current existenting vaccines. In fact, the question of the RNA structure analysis was not covered as thoroughly as others, but the technological basis behind the vaccines is adequately described.
In order to estimate the effectiveness of the different types of vaccines, it is essential to deliver information about the structure, genome, and mode of infection of SARS-COV-2. Fortunately, I examined numerous studies that were concerned with this topic. These researches come predominantly from the scholarly article from Rokni et al. (2020), who explicitly explained the virus’s composition. Several different works are worth mentioning in the context of the SARS-COV-2 structure: Varga et al. (2020), Covián et al. (2020), and Xia et al. (2021). The last research was done by professionals in cell biology that are not USA or European citizens. Yet, they used the English language in their work, which helped regain more diverse data for our research.
The four different vaccines were compared and evaluated with the help of several scales. The sources for evaluation comprised Thompson et al. (2021), Vogel et al. (2021), Kowarz et al. (2021), and Zimmer et al. (2021). These journals and articles were included because of their recent publication dates and relatively well-described statistical methods and references. Moreover, we assumed it is significant to mention various approaches to the mentioned authors’ population samples.
With the emergence of coronaviruses’ mutations, it is vital to keep updates about the vaccines’ reactions to the changes of the pathogen they are aimed to prevent from spreading. The Delta variant of the virus has been developing recently, so that the literature about its structure and interaction with vaccines was expected to be scarce. It could be concluded that the current state of knowledge about the emerging threat from novel coronavirus variants is not yet satisfying.
Finally, the research attempted to assess the safety of the four vaccines. Since there is only limited quantitative data about this kind of information, I decided to describe the measures of safety envisaged by the vaccines’ designers and the risks shown by the negative cases of vaccination. For the former suggestion I used the general description of the vaccines’ works performed by Graham et al. (2020). The probable adverse effects of particular vaccines are discussed in Kowarz et al. (2021).
Results
The scholars thoroughly described the four most popular and scientifically approved vaccines and referenced them in the popular journals with news-structured content. The situation is the same with the description of the coronaviruses: these are observed and studied in great detail. However, the problems of the vaccines’ safety, effectiveness against the new delta variant of coronavirus, and the exact mechanism of the RNA structure relation are not researched on the same high level as the previous topics.
The research showed what the available online information reveals about the pandemic, its causes, and prevention. The virus develops rapidly and produces new mutations that cause an even more destructive impact on people. The dominant method of reducing the adverse outcomes of the viruses’ spread is vaccination, supported by the academic sphere and governments of the USA and Europe. The existing two types of vaccines work with different levels of effectiveness; in most cases, they are safe for use but cannot handle the Delta variant of the virus with much effectiveness. The research shows mixed results of informativeness concerning different aspects of the problematic situation with the disease caused by the virus.
Discussion, Implication and Conclusion
In the early stage of research, it was discovered that there are no comprehensive overviews of the current situation connected to the spread of the COVID-19 disease. The current published peer-reviewed scholarly articles contain information about various areas of the problem. Yet, no general analysis and extensive data processing have been conducted. This lack of systematic evaluation is due to the emergency of the issue and the novelty of the problem. Currently, the majority of researches are concerned with specific data about coronaviruses and vaccines. Thus, the fragments of knowledge about the vaccination have been gathered in this study to present a holistic understanding of the problem in one paper.
Discussion
Some aspects of the research were not covered explicitly for several essential reasons. The first is that scientists are still understanding some of the characetristics of different COVID-19 variants and their efficacy, relative to current vaccines. Part of the problem is that viral infections have not been damaging to all the spheres of human activities. Despite the fact that the issue is overwhelming and important, it primarily serves more of a theoretical purpose that primarily affects the vaccines’ effectiveness. It may be challenging to discern this matter among others when a practical solution determines the priorities of the researches. Moreover, the vaccines are developed relatively recently, which indicates that data about them is only beginning to emerge. In the foreseeable future, there would probably be more considerations on the exact mechanisms involved in the work of vaccines.
Next, the effect of the Delta variant of coronavirus is not yet studied in the same exact matter as other types. Obviously, it is caused by the recentness of its emergence while the vaccines aimed to prevent the already well-observed viruses. The four types of vaccines serve as an immediate solution for the existing problem and demonstrate high effectiveness if the conditions in which they have been developed are considered. The effectiveness of the preventive methods against the Delta virus is not well-studied, specifically in the USA and Europe. Currently, medical organizations publish guidelines on preventing the spread of the new Delta variant, which is the most appropriate option in the conditions of limited comprehension of the issue.
In turn, the majority of questions discussed in the research have answers in multiple journals and databases. This notion is important because it demonstrates the high efforts of researchers worldwide to develop an effective solution for the COVID-19 pandemic. Predominantly, the information is published on the platforms with free access and online, which is evident in the united attempt to share knowledge about the most crucial problem nowadays. Moreover, the data is accurate, specific, and illustrative for the purpose of shared understanding of the nature and outcomes of the COVID-19 infection. Hence, the global scholar community displayed an outstanding level of cooperation while faced with such a pandemic.
Implication
The research is valuable for the scientific community and adult learners profoundly interested and concerned about the current state of knowledge of the development and effectiveness of the COVID-19 vaccines. The general audience can investigate the nature of the current global COVID-19 problem since the language of the research is concise and comprehensible. Moreover, the study presents a short overview of the data/information instead of a detailed evaluation which may be more insightful for the majority of the English-speaking population. Yet, the sources are also mentioned so that the research’s credibility may be estimated accordingly. Also, the sources mentioned are entirely free and could be accessed even by those with no available resources for gaining scientific articles. Therefore, one of the important implications of the research is its openness to an average English-speaking reader.
The other goal of the current research is to demonstrate the gathered data for scholars as well as recognize the issues in the state of knowledge of the problem. The research contains a brief analysis of multiple sources on the specific range of questions, yet under a broad topic. The literature review provided by the survey may be helpful for a quick overview of the most relevant information about the vaccines and the COVID-19 itself. Moreover, even the limited scope of the research demonstrated that there are areas that need further detailed research. For example, it is apparent that the problem of the relation between RNA structure and the four particular vaccines is essential but not appropriately surveyed. Next, the vaccines must be constantly evaluated for their effectiveness and safety to make some improvements. Yet, the answer to the question of safety can be found only in researches that aim to explain other concepts. Thus, the issue should be resolved directly. The situation is similar to the challenge that emerged because of the Delta variant of the coronavirus. The vaccines must be improved due to the mutations that begin to affect their effectiveness. Otherwise, a new surge of infection cases may result in even more dramatic consequences for humanity. Hence, the field of study that concerns the immunology related to the coronavirus problem should consider additional topics for research.
Conclusion
One of the main threats to the international health system remains the new COVID-19 coronavirus infection caused by the SARS-CoV-2 virus. SARS-CoV-2 is a virus of natural origin and is well-studied; yet, the coronaviruses develop mutations that present challenges to the scientific world. There are two different vaccine technologies to prevent the spread of the disease with a probability of a mortal outcome. In vector-based adenovirus vaccines, the drug injection contains third-party viruses that cannot replicate and are equipped with copies of the SARS-CoV-2 genes. The list of such vaccines includes AstraZeneca/Oxford and Johnson & Johnson. They enter the immune cells and trigger the mechanisms for starting the biosynthesis of the S-protein of the coronavirus. On the alternative side, there are mRNA vaccines that contain an RNA molecule. Vaccines such as Pfizer-BioNTech and Moderna deliver ready-made instructions to target cells to synthesize a protein antigen, in response to which the human immune system produces its antibodies. Both vaccines work effectively, although the first type is more compact and can help the population, which has a higher risk of adverse reaction on the vaccines. Some scholars debate the safety of vaccines since certain features of the medications can produce harmful effects on the health of vaccinated patients. This aspect and the scientific basis between the RNA structure and its linkage to the vaccines should be studied by researchers thoroughly.
References
Boopathi, S., Poma, A.B. and Kolandaivel, P. (2021). Novel 2019 coronavirus structure, mechanism of action, antiviral drug promises and rule out against its treatment.Journal of Biomolecular Structure and Dynamics, 39(9), 3409-3418. Web.
Bose, P. (2021). Omicron variant may strike double vaccinated. Web.
CDC. (2021). Omicron variant: What you need to know. Web.
Chang J. (2021). Adenovirus vectors: excellent tools for vaccine development.Immune network, 21(1), 6. Web.
Covián, C., Ríos, M., Berríos-Rojas, R. V., Bueno, S. M., & Kalergis, A. M. (2020). Induction of trained immunity by recombinant vaccines. Frontiers in Immunology, 11, 1-9. Web.
Creative Biolabs. (2021). Adenovirus (Ad) as vaccine-vectors. Web.
Cross, R. (2020). The tiny tweak behind COVID-19 vaccines. Web.
Disantara, F. P. (2020). The large scale social restrictions policy for handling the Covid-19 pandemic.Jurnal Pembaharuan Hukum, 7(2), 128-141. Web.
Garde, D. (2020). The story of mRNA: How a once-dismissed idea became a leading technology in the COVID vaccine race.
Graham, S. P., McLean, R. K., Spencer, A. J., Belij-Rammerstorfer, S., Wright, D., Ulaszewska, M.,… & Lambe, T. (2020). Evaluation of the immunogenicity of prime-boost vaccination with the replication-deficient viral vectored COVID-19 vaccine candidate ChAdOx1 nCoV-19. npj Vaccines, 5(1), 1-6. Web.
Guevara, M. L., Persano, F., & Persano, S. (2020). Advances in lipid nanoparticles for mRNA-based cancer immunotherapy.Frontiers in Chemistry, 8, 963-980. Web.
Hackethal, V. (2021). Here’s why viral vector vaccines don’t alter DNA. Web.
Hou, X., Zaks, T., & Langer, R. (2021). Lipid nanoparticles for mRNA delivery. Nature Reviews Materials, 6(2), 1078–1094.
Inagaki, H., Saito, A., Kaneko, C., Sugiyama, H., Okabayashi, T., & Fujimoto, S. (2021). Rapid inactivation of SARS-CoV-2 variants by continuous and intermittent irradiation with a deep-ultraviolet light-emitting diode (DUV-LED) device. Pathogens, 10(6), 754-762. Web.
Kindrachuk, J., & Shaw, S. (2021). COVID-19 Delta variant in Canada. Web.
Kowarz, E., Krutzke, L., Reis, J., Bracharz, S., Kochanek, S., & Marschalek, R. (2021). “Vaccine-Induced Covid-19 Mimicry” Syndrome: Splice reactions within the SARS-CoV-2 Spike open reading frame result in Spike protein variants that may cause thromboembolic events in patients immunized with vector-based vaccines. Journal of Experimental & Clinical Cancer Research, XX, 1-24. Web.
Lainscek, D., Fink, T., Forstneric, V., Hafner-Bratkovic, I., Orehek, S., Strmsek, Z.,… & Jerala, R. (2020). Immune response to vaccine candidates based on different types of nanoscaffolded RBD domain of the SARS-CoV-2 spike protein [PDF document]. Web.
Mendonça, S.A., Lorincz, R., & Boucher, P. (2021). Adenoviral vector vaccine platforms in the SARS-CoV-2 pandemic. NPJ Vaccines, 6(2), 97, 1-11.
Morais, P., Adachi, H., & Yu, Y. (2021). The critical contribution of Pseudouridine to mRNA COVID-19 vaccines. Web.
Nance, K. D., & Meier, J. L. (2021). Modifications in an emergency: The role of N1-Methylpseudouridine in COVID-19 vaccines. ACS Central Science, 7(5), 748–756.
Pancevski, B. (2021). Blood expert says he found why some Covid-19 vaccines trigger rare clots. WSJ. Web.
Rokni, M., Ghasemi, V., & Tavakoli, Z. (2020). Immune responses and pathogenesis of SARS‐CoV‐2 during an outbreak in Iran: Comparison with SARS and MERS. Reviews in Medical Virology, 30(3), 1-6. Web.
Servick, K. (2020). This mysterious $2 billion biotech is revealing the secrets behind its new drugs and vaccines. Web.
Taib, S. D. M. B. M., & Alib, H. S. S. (2021). Genetic variation and evolution of the 2019 novel coronavirus. Public Health Genomics, 24(1-2), 1-13. Web.
Tenchov, R., Bird, R., Curtze, A., & Zhou, Q. (2021). Lipid nanoparticles─from liposomes to mRNA vaccine delivery, a landscape of research diversity and advancement. ACS Nano, 15(11), 16982–17015.
Thompson, M. G., Burgess, J. L., Naleway, A. L., Tyner, H. L., Yoon, S. K., Meece, J.,… & Gaglani, M. (2021). Interim estimates of vaccine effectiveness of BNT162b2 and mRNA-1273 COVID-19 vaccines in preventing SARS-CoV-2 infection among health care personnel, first responders, and other essential and frontline workers—eight US locations, December 2020–March 2021. Morbidity and Mortality Weekly Report, 70(13), 495-500. Web.
Varga, Z., Flammer, A. J., Steiger, P., Haberecker, M., Andermatt, R., Zinkernagel, A.,… & Moch, H. (2020). Electron microscopy of SARS-CoV-2: a challenging task–Authors’ reply. The Lancet, 395(10238), 1.
Venkadapathi, J., Govindarajan, V. K., Sekaran, S., & Venkatapathy, S. (2021). A minireview of the promising drugs and vaccines in pipeline for the treatment of COVID-19 and current update on clinical trials. Frontiers in Molecular Biosciences, 8, 1-7. Web.
Vogel, G., & Kupferschmidt, K. (2021). Side effect worry grows for AstraZeneca/Oxford vaccine. Science, 372(6537), 14-15.
Woodruff, M. C., Ramonell, R. P., Nguyen, D. C., Cashman, K. S., Saini, A. S., Haddad, N. S.,… & Sanz, I. (2020). Extrafollicular B cell responses correlate with neutralizing antibodies and morbidity in COVID-19. Nature Immunology, 21(12), 1506-1516. Web.
World Health Organization. (2021). Tracking SARS-CoV-2 variants. Web.
Xia, B., Shen, X., He, Y., Pan, X., Liu, F. L., Wang, Y.,… & Gao, Z. (2021). SARS-CoV-2 envelope protein causes acute respiratory distress syndrome (ARDS)-like pathological damages and constitutes an antiviral target. Cell Research, 31(1), 1-14. Web.
Xie, J. (2021). Revealed: The protein ‘spike’ that lets the 2019-nCoV coronavirus pierce and invade human cells. Web.
Zimmer, C., Weiland, N., & LaFraniere, S. (2021). New analyses show Johnson & Johnson’s one-dose vaccine works well. NY Times. Web.