Clinical Trials
Conducting a safe and reliable laboratory research on children is a remarkably important issue of modern medicine, since in recent years high automated technologies of many manufacturers of diagnostic tools have entered practical laboratory diagnostics, the list of laboratory tests has increased, patients have the opportunity to be examined in various healthcare institutions, which may be in different areas, cities, countries.
The concept of exclusivity can be considered as exclusive marketing right, which is given by FDA, and it can be run with a patent (Patents and exclusivity, 2015).
In the US, the central role in this area belongs to the FDA Office of Orphan Products Development (OOPD), which primarily focuses on fostering safe and effective drug development (About orphan products clinical trial grants, 2018). The overall clinical trial design needs to include planning, drug disposition, sampling strategy, drug response analysis (Shakhnovich et al., 2019).
Therefore, it is necessary to minimize the discrepancy or bias in the data obtained during the research by means of inter-laboratory comparisons and regression analysis, which should be carried out promptly, as necessary, by available means and at convenient time periods.
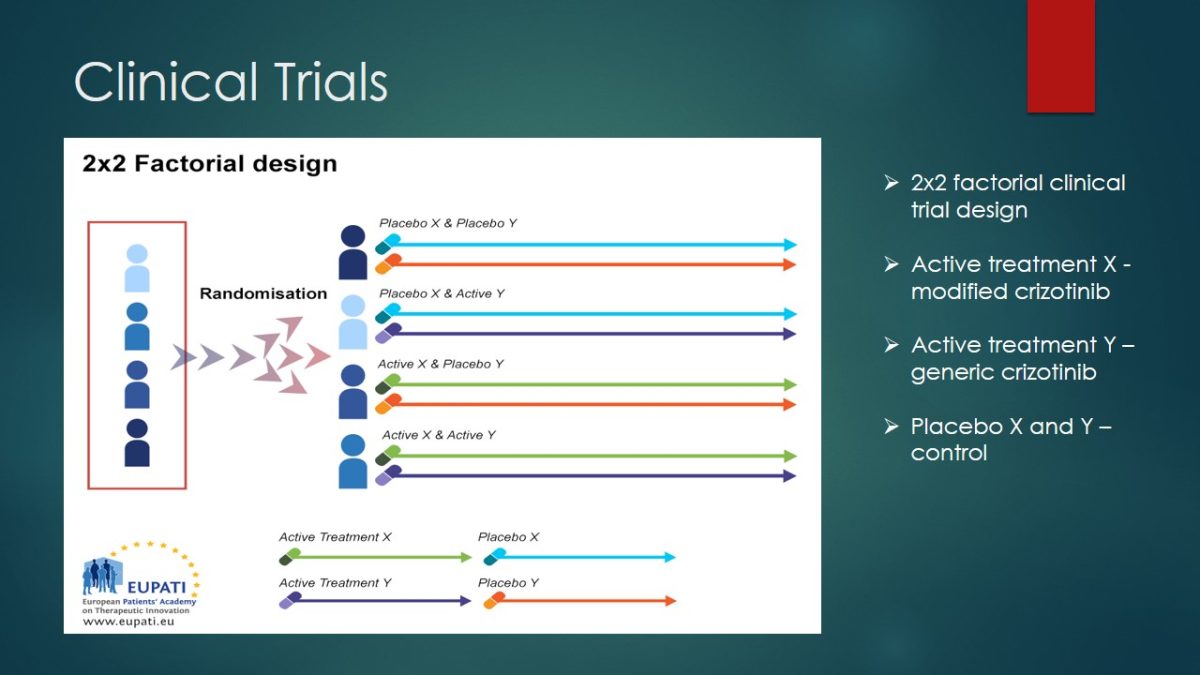
- 2×2 factorial clinical trial design.
- Active treatment X – modified crizotinib.
- Active treatment Y – generic crizotinib.
- Placebo X and Y – control.
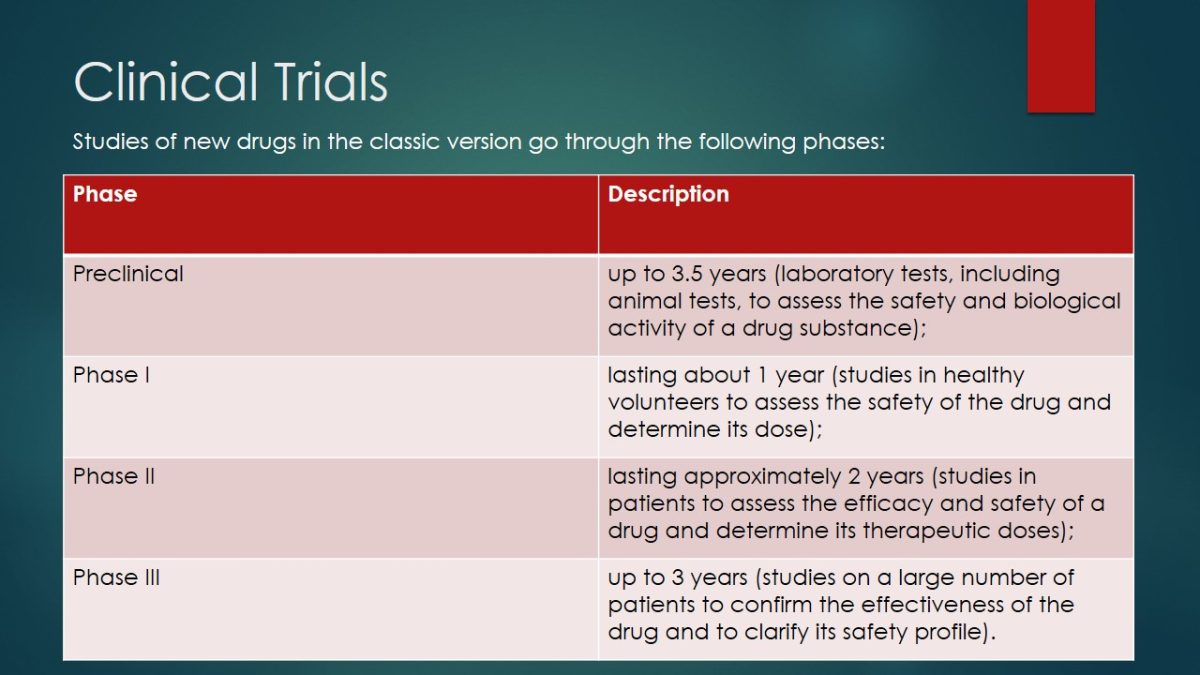
Studies of new drugs in the classic version go through the following phases:
The information collected during clinical trials is analyzed, and then documents for the registration of the drug are submitted.
Before registering a drug, regulators – for example, the US Food and Drug Administration (FDA) – carefully review the results of clinical trials, and if necessary, check the quality of the study and the accuracy of the data presented.
With a negative assessment of the results of studies, the drug cannot be registered.
After registration of the drug, clinical studies can be continued: post-registration studies of phase IV are carried out, the main purpose of which is to identify and determine previously unknown or incorrectly evaluated side effects of the drug, as well as risk factors at the population level.
It should be noted that at present, there is a tendency to blur the boundaries between different phases of clinical trials, along with simultaneous studies of different phases.
- Phase I – children with oncology are allowed to participate in clinical trials;
- Phase II – uncommon for most drugs in pediatrics, but acceptable for drugs to treat cancer, including crizotinib;
- Phase III – acceptable to test drugs in comparison to other therapies in children with oncology.
For the development of a new formula for crizotinib to be applied in the pediatric population, the focus of clinical trials should be on Phase II and Phase III for the selected population and cancer type. In pediatrics, the development of drugs to treat cancer allows for the normal involvement of subjects and the shift to Phase II and Phase III to promote the further drug testing. Thus, Phase II for testing safety is usually uncommon for most drugs in pediatrics, except for drugs to treat cancer, including crizotinib. Phase III is for testing the drug effectiveness in comparison to other therapies, and it is acceptable to test drugs in children with oncology.



Phase II
- At Phase II, crizotinib will be taken by the pediatric population under the control of researchers until the possible disease progression or until observing toxicity.
- Clinical responses related to the possible progression of the disease, changes in the tumor, or toxicity and other side effects will be assessed after each 10 days of treatment.
- Subjects who will complete all the treatment cycles without observed signs of the disease progression and negative side effects will be shifted to Phase III.

Phase III
- At Phase III, crizotinib will be taken by one group of the participants under the control of researchers, and the other group of the participants will undergo their usual therapy.
- Results of therapies and possible changes in the disease progression will be assessed each 24 days of treatment.
- The response rate to the changed crizotinib will be evaluated.
- Participants who have certain comorbidities or whose state has worsened since the previous phase need to be excluded from the study.
- Currently, pediatrics does not have a sufficient arsenal of drugs officially approved for use in childhood.
- For most childhood diseases, there are as yet no special pediatric drugs, there is extremely little data on the safety of the use of drugs in children and clearly not enough specific pediatric dosage forms.
- In the absence of clinical studies, the vast majority of young patients receive drugs unregistered for a given age.
- The shortage of medicines intended specifically for use in children forces pediatricians to take risks using drugs that are not registered for the treatment of children.
- This risk is especially increased in diseases of early childhood, as well as in severe diseases rarely found in children.
- Thus, the majority of drugs prescribed for newborns are not registered for use in this age group.
- However, the use of some potentially effective drugs in children is unreasonably delayed indefinitely.
- It is important to note that the method of quality control of clinical laboratory research at the level of the healthcare system is based on the processing of results carried out by clinical diagnostic laboratories examining samples of control materials sent by the center for external quality control of clinical laboratory research and its regional branches.
- It is also stated that the process of estimating the degree of effect can play a significant role in providing a clinical benefit (Rare diseases: Common issues in drug development, 2019).
- The purpose of conducting and assessing the quality of research is to assess the degree of comparability of the results of research performed in various healthcare institutions and their compliance with the standards of analytical accuracy established by regulatory documents.
- In addition, it is critical to define that for pediatric patients the dose is crizotinib 280 mg/m2 (Crizotinib (Xalkori) expanded access protocol for the treatment of adult or pediatric patients, 2015).
- Participation in events is mandatory for laboratories of healthcare institutions of all forms of ownership and is taken into account during their accreditation and licensing.
- It is important to understand that control is carried out according to several indicators of laboratory tests on one or two control samples of a factory-made analysis outcomes with the participation of clinical diagnostic laboratories of all units.
- The primary objective of pediatric clinical trials is to derive the maximum tolerated dosage (MTD) (Crizotinib in treating younger patients with relapsed or refractory solid tumors or anaplastic large cell lymphoma, 2020).
- The target clinical trials are designed to address pediatric non-small cell lung cancer, which means the common symptoms, such as shortness of breath and hemoptysis, needs to be evaluated (Pediatric non-small cell lung cancer, 2019).
- In each participating laboratory, each sample is measured several times, then the average value of the two results is calculated and the results are sent to testing centers, where they are processed on a computer using standardizing methods and calculating the statistical parameters of average values, standard deviation and coefficient of variation.
- As a criterion for comparing and evaluating the correctness of the results, average values of indicators obtained from all participating laboratories are used.




Regulatory Implications
- The team selected the option of developing a new drug, thus, applying for new patent is required.
- Patent gives 20 years total. It can be acquired before drug approval, thus effective patent term is less than 20 years.
- Exclusivity is granted separately and it can only be gained after drug approval.
- Orphan drug exclusivity is equal to 7 years, whereas pediatric exclusivity adds 6 months to the already acquired exclusivity (Frequently asked questions on patents and exclusivity, 2020).
- In Europe, if during the first eight years there are substantial clinical benefits, the ten-year exclusivity can be extended to eleven years total (Data exclusivity, market protection, orphan and paediatric rewards, 2018).
- In order to apply for exclusivity the following steps are needed:
- Patent information is submitted with new drug applications (NDAs);
- Form FDA 3542a is filled at the time of submission of NDA;
- Within 30 days of approval, Form FDA 3542 patent must be listed in the Orange Book;
- If the forms are not filled correctly, 15 days are granted for corrections (Frequently asked questions on patents and exclusivity, 2020).
- The approval process requires Quality Control information.
- The option of developing new version of crizotinib, which will require new patent.
- It is important to note the fact the outcome can have similar pattern as in previous trails. For instance, the results show that the overall survival is 49%, whereas progression free survival is equal to 82% (Davis et al., 2018).
- Thus, market exclusivity can be declined.



Reference List
About orphan products clinical trial grants (2018). Web.
Crizotinib in treating younger patients with relapsed or refractory solid tumors or anaplastic large cell lymphoma (2020). Web.
Crizotinib (Xalkori) expanded access protocol for the treatment of adult or pediatric patients (2015). Web.
Data exclusivity, market protection, orphan and paediatric rewards (2018). Web.
Davis, K. L. et al. (2018) ‘Real-world outcomes in patients with ALK-positive non-small cell lung cancer treated with crizotinib’, Current Oncology, 25(1), pp. 40-49.
FDA (n.d.) Resources. Web.
Frequently asked questions on patents and exclusivity (2020). Web.
Pediatric non-small cell lung cancer (2019). Web.
Patents and exclusivity (2015). Web.
Rare diseases: Common issues in drug development (2019). Web.
Shakhnovich, V. et al. (2019) ‘How to conduct clinical trials in children: A tutorial’, Clinical and Translational Science, 12(3), pp. 218-230.
2×2 factorial design. Web.