Abstract
The Pancreas in a chimpanzee is an endocrine and exocrine organ involved in the metabolism of day-to-day body operations. The exocrine pancreas produces digestive enzymes and juices that facilitate the digestion of proteins, lipids, and carbohydrates by creating an appropriate medium for enzymes function and providing the enzymes to break down these food products. Some animals have discrete endocrine and exocrine pancreas but for higher animals like chimpanzees, the two are usually fused. This review will show the development of the chimpanzee pancreas, the anatomy of the fully developed pancreas including its histological cellular components, the molecular interactions that regulate the development of the pancreas, and discuss a developmental abnormality in the embryology of the pancreas.
Introduction
Two haploid cells (sperm and ova) fuse during fertilization in the ampullae of fallopian tubes leading to a cascade of events that culminate in the formation of a fully developed fetus resembling a chimpanzee at the end of gestation. Thirty hours later two cells are formed, forty hours later a four-cell stage, 72 hours later you have 12 – 16 cells and a late morula is formed by day four. This morula is implanted by day six and blastocyst with embryoblast and trophoblast is formed by the end of week one.
The formation of a bilaminar disc is the main event in week two. It has two cell layers (hypoblast and epiblast) and two cavities (amniotic cavity and primitive yolk sac). Week three is heralded by gastrulation with the formation of the trilaminar germ disc that is composed of the epiblast, endoderm, mesoderm, and ectoderm. All the organs of the body originate from these three layers (Cleaver and Krieg 9)
Week three to eight form the period of organogenesis and is the most vulnerable to teratogens. The cephalocaudal and lateral folding of the trilaminar disc leads to the formation of the primitive gut from the yolk sac. The two cavities that remain outside form the allantois and the yolk sac and these communicate with the primitive gut via the vitelline duct. These mesenteries give peritoneal ligaments which carry the neurovascular bundles and lymphatic of the developing gastrointestinal organs. The part of the primitive gut in the cephalic end becomes the foregut and the caudal part becomes the hindgut with the middle portion being the midgut,
Embryology of the Pancreas
The pancreas develops from the endodermal lining of the caudal part of the foregut that forms the duodenum. Two buds pouch from the endoderm; a larger bud, the dorsal pancreatic bud appears first in the dorsal mesentery followed by a smaller bud, the ventral pancreatic bud that appears in close relation with the bile duct. The duct system and parenchyma develop in each bud then fuse to form one organ. This results in the formation of the principal pancreatic duct of Wirsung. The proximal part of the dorsal pancreatic bud may become atretic and obliterated or persist as the accessory pancreatic duct of Santorini drains into the duodenum through the minor papilla. About 10% fail to fuse leading to two drainage systems each draining to the closest portion of the gut.
The connective tissue of the pancreas is formed from the surrounding splanchnic mesoderm. The rotation of the stomach, ventral mesentery, and the duodenum push the pancreas to lie in the stomach bed in the posterior abdomen. The pancreas is pushed up by the transverse colon and its peritoneum fuses with that of the posterior abdominal wall making the pancreas a retroperitoneal organ in the stomach bed.
During the third month of fetal life, the parenchymatous tissue of the pancreas gives rise to islets of Langerhans (discrete cell aggregates derived from endoderm) which are scattered throughout the pancreas. Glucagon, Somatostatin and pancreatic polypeptide secreting cells also develop from the parenchyma. This leads to the formation of the endocrine portion of the pancreas with insulin secretion beginning as early as the fifth month of fetal life (Moore and Torchia 23).
Molecular Interactions in the Development of the Pancreas
Several interactions take place to ensure the body organs develop and in an orderly sequence in the right location. The development of the gut tube is regulated by reciprocal interaction of the endoderm and the surrounding splanchnic mesoderm. This interaction allows orderly differentiation of the gut and its derivatives. HOX code resulting from the expression of the sonic hedgehog gene establishes the cephalocaudal axis of the gut (Jensen 180).
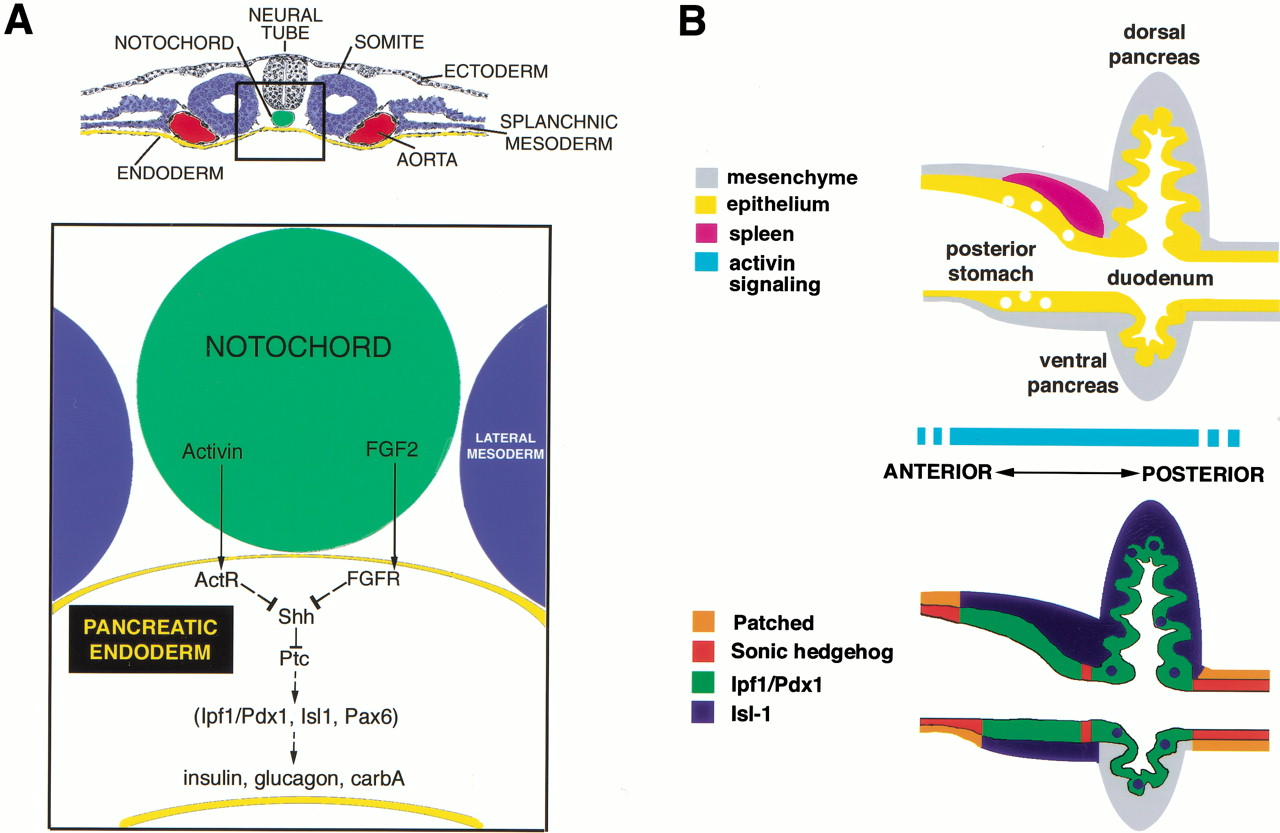
Fibroblast growth factor (FGF) and activin (a family member of the TGF-B family) play a main role in the initiation of pancreas development. They are produced by the notochord and repress expression of the SHH in the gut mesoderm leading to up-regulation of the pancreatic and duodenal homeobox 1 (pdf) gene. This notochord endodermal interaction induces transcription of pancreatic marker genes allowing pancreas development together with spatial restricted expression of the Hox transcriptional factors which regulates the anterior-posterior gastrointestinal pattern.
The pancreatic architecture, which plays a major role in pancreatic diseases, is tightly regulated by the interplay of FGF, Activin, and hedgehog signaling. The fate of pancreatic cells is regulated in a way that leads to about 95-99% of the cells being exocrine and the remaining being endocrine. The mesenchymal signals have been shown to promote the exocrine pancreas and if removed lead to impaired exocrine development but normal endocrine development. Follistatin also promotes the exocrine pancreas development by inhibiting TGF-B and activin. TGF-B, activin, and intracellular Smads like Smad 2 &4 expressed in the pancreatic epithelium and mesenchyme promote the development of the endocrine pancreas and regulate the islet size (Jensen 180).
Islet cells expressing paired PAX 4 & PAX 6 become insulin, Somatostatin, and pancreatic polypeptide producing while the islets that express only PAX 6 become alpha cells producing glucagon. These degrade the basal membrane and extracellular matrix molecules. Immediately after migration, there is a reorganization of the endocrine cells resulting in the distinguishing islets architecture. This phenomenon is modulated by cell adhesion molecules (CAMs) such as cadherins.
The endoderm expresses some transcriptional factors required for the development and function of the pancreas. The Ipf1/pdx1 when not expressed, as the case in mice without homeodomain transcriptional factor H1xb9, result in agenesis of the dorsal pancreas and abnormal islets development in the ventral pancreas. The regulation of the Hox and ParaHox gene products like Ipf1/pdx1 is by PBX 1 and the Meis-TALE class homeodomain proteins. Mutations affecting the receptors for activin (Act RIIA & B) disrupt pancreatic development as well as that of other gut derivatives like stomach and spleen.
Anatomy of the Fully Developed Pancreas
The pancreas is an intra-abdominal retroperitoneal organ in location. It occurs in the transverse axis along the transpyloric line. The massive larger section occurs in the left hypochondriac region. Due to its retroperitoneal location, this organ lies along the posterior abdominal wall taking a long irregular prismatic shape approximately 12.5cm to 15 cm long. Its weight can vary from 60gm to 100 gm.
The pancreas is made up of the head, uncinate process, and neck. Furthermore, it has a body and a tail.
Relations of the Pancreas
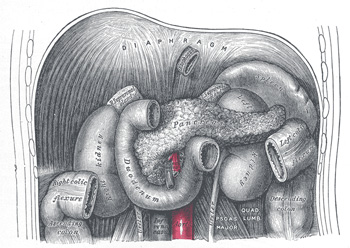
- Head: lodged within the C-shaped curve of the duodenum, it superiorly overlaps the upper part of the duodenum and inferiorly overlaps the lower horizontal part. Interiorly lies the transverse colon with an intervening areola tissue. The superior mesenteric artery and vein run anterior to its left half. Posterior to it lies the inferior vena cava, the renal veins, the common bile duct, aorta, and the right diaphragmatic crura.
- The Body: interiorly is the posteroinferior surface of the stomach with an intervening omental bursa separating the two organs. Inferiorly there is duodenal-jejunal flexure with the extreme left relating to the left colic flexure. The superior surface lies about the celiac artery and the diaphragm.
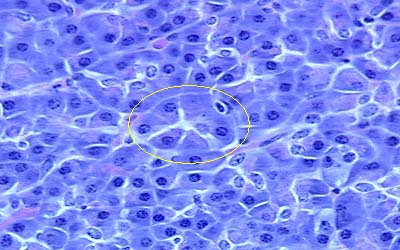
The Histology of the Pancreas
Microscopically, the pancreas is structurally related to other endocrine and exocrine glands more so the salivary gland. Of note is that this gland has got an endocrine and exocrine component hence its classification as a compound gland. Histologically, the pancreatic gland is made up of lobules which consist of one of the ultimate ramifications of the main duct, ending distally in many alveoli lines with secretory columnar epithelium. These alveoli are separated by connective tissue elements which at some points presents islands of cells called islands of Langerhans lies juxtaposed to numerous capillaries into which they secrete hormones. These contain the two types of cells participating in the pancreas’ endocrine function namely: A-cells secreting glucagon and B-cells that secrete insulin (Mfopou, Bing, Lina, Karen and Luc 2100).
The exocrine pancreas is therefore composed of secretory cells clustered together in a grape-like pattern to form the acinus into which the secretory cells empty their contents. Intercalated ducts receive the secretions from the acini and coalesce within the lobule to form the intralobular ducts. These, in turn, converge to form the interlobular ducts that eventually form the main pancreatic duct. This pancreatic duct runs within the substance of the gland to join the bile duct before its emptying in the second part of the duodenum (Scott 45).
Developmental Anomalies
Most anatomical abnormalities arise from ineffective or defective embryological development and the resulting presenting symptoms vary from patient to patient depending on the organ affected.
These abnormalities tend to remain asymptomatic in the majority of cases whereby they are incidentally detected during investigative procedures involving abdominal viscera. However, in some cases, symptoms may be present and life-threatening.
Annotated Pancreas
This is an odd defect manifested by a loop of pancreatic tissue. Etiologically, it’s postulated that the ventral pancreatic Anlagen divides too early during embryological development into two segments that develop separately. As mentioned earlier, this anomaly is an incidental finding in a majority of chimpanzees. However, in the other minority, the annular pancreas has been associated with the following complications. Duodenal stenosis associated with intestinal obstruction often requires surgical intervention. The section of the duodenum surrounded by the pancreatic tissue undergoes narrowing of the lumen with an ensuing impedance of the normal transit of the gut contents from the stomach into the small intestines.
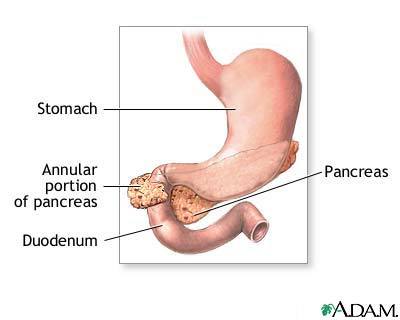
Other complications are biliary obstruction, chronic pancreatitis, peptic ulcers, peritonitis and intestinal perforation (Lewis, Raff, Roberts, and Peter 344). Other developmental anomalies may occur.
Works Cited
Cleaver, Oliver, and Krieg, Petersen. Notochord patterning of the endoderm. Developmental Biology 234.1 (2001): 1–12.
Jensen, Jorger. Gene Regulatory Factors in Pancreatic Development. Developmental Biology 229.1 (2004): 176–200.
Moore, Keith, and Mark, Torchia. Clinically oriented embryology. New York: Elsevier, 2008.
Lewis, Julian, Raff, Martin, Roberts, Keith, and Walter, Peter. Molecular Biology of the Cell. 4th ed. New York: Garland Publishing, 2002.
Mfopou, Josué, Bing, Chen, Lina, Sui, Karen, Sermon, and Luc, Bouwens. Recent Advances and Prospects in the Differentiation of Pancreatic Cells from Embryonic Stem Cells. Diabetes 59.9 (2010): 2094-2101.
Nussey, Simon, and Whitehead, Andrew. Endocrinology: An Integrated Approach. Oxford, UK: BIOS Scientific Publishers Ltd, 2001.
Scott, Gilbert. Developmental Biology. 9th ed. New York: Sinauer Associates Inc., 2010.