Introduction
Wastewater is fundamentally designated as the flow of used water discharged from businesses, institutions, commercial tasks, homes, and firms, directed to a treatment plant(s) by an accurately designed and engineered system of pipes. The wastewater is also categorized and characterized according to its source of origin. Wastewater management is a major issue at both industrial and domestic levels that needs to be resolved for access to quality water for use at both levels. It noted the amount of wastewater generated by a single individual daily amounts to about 200- 500 liters daily. This paper will thus evaluate the two superior methods of wastewater management and treatment, which are composed of: biological and chemical methods.
Situation and Problem
The wastewater treatment issue is a fundamentally critical topic of discussion in the public domain in that: it is the public that experiences the use of contaminated water, experiences water shortage, and the inability to recover it.
Gross violation of the existing water acts by the providers also felt by the public as well as increasing health and medical issues such as, Cholera which threatens the life of the citizens.
The ever-increasing demand for water by the ever-rising population is experienced too as the public uses any water sources at their disposition, leading to faster depletion. The major solutions to wastewater treatment thus include:
Solution 1: Chemical Treatment Method
Generally, it is credible to appreciate the fact that a general wastewater treatment plant undergoes six major steps that are undertaken for the full treatment process to be achieved. The steps are composed of: Preliminary treatment, which focuses on the removal of
the solid materials (visible), which can spark an operational problem. The Primary stage removes about 60% solids and about 35% of the biochemical oxygen demand (BOD), as argued by DEFRA.
The other essential stages are: the Secondary treatment which focuses on removal of about 85% of the biochemical oxygen demand (BOD) and any remaining solids, the advanced treatment stage which focuses on removal of about 95% of the BOD and any present solids as well as final treatment and the sludge management process. The major conceptual stages in the wastewater plant layout are as shown by the figure below.
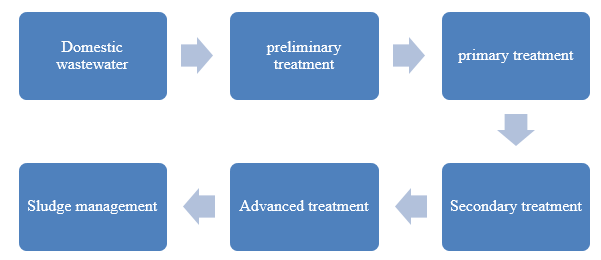
The chemical wastewater treatment process focuses on the removal of the present pollutants, which are not readily removed by the physical process. These pollutants are composed of: suspended solids, BOD (usually in the range of 10-15 milligrams per liter), heavy metals present such as Cadmium, refractory organics, and any inorganic salts.
The application of the Chemical treatment method is a result of the inability of both the secondary and primary steps to remove some microscopic elements such as; phosphorous, Cadmium, Mercury, or even Zinc, which exists as trace elements in wastewater.
Solution 2: Biological Method
DEFRA demonstrates that the wastewater treatment process in most cases precedes as a biological operation- a tertiary process that is composed of the chemical precipitation, neutralization, adsorption, ion exchange, and disinfection through use of Chlorine, Ozone or even Ultraviolet light.
The first technique of chemical wastewater treatment is the chemical precipitation, which involves the addition of a dissolved inorganic compound, to either a base or an acid, by ensuring that the temperature is adjusted.
The second approach in the wastewater treatment operations in chemical operation is the neutralization operations, which involve the control of the pH of the wastewater, which may either be acid or basic to a neutral PH of 7. If the wastewater is acidic in most cases, there is an addition of a base such as; calcium hydroxide (Ca (OH) 2), calcium oxide (lime) (CaO), sodium hydroxide (NaOH), or sodium carbonate (Na2CO3) generally known as soda ash.
On the other hand, if the wastewater realized to be too basic, some of the acids added to adjust the PH to 7 of the wastewater include the carbonic acid (H2CO3) and the sulphuric acid (H2SO4).
The use of the Ion-exchange catalytic operation also applied as a chemical operation in the treatment of the wastewater. In this process, a reversible reaction takes place in which a charged ion from a solution of wastewater exchanged with a charged ion that has an electrostatic charge attached to an immobile phase. The application of this chemical process in the treatment of the wastewater is majorly projected to soften the water, mainly exchanging the polyvalent cations such as Magnesium or Calcium with the sodium ion.
The engineering of the exchange resins in most cases designed by the use of the polymer organic compounds whereby sodium from the ion exchanger exchanged with cations in the wastewater solution until the bed is saturated.
The application of Ozone as an oxidant also applied as a chemical method for the treatment of the wastewater. This is through the ability of the Ozone gas to oxidize a wide range of both organic and inorganic compounds during wastewater treatment leading to clean water. The use of Ozone in wastewater treatment fulfilled through the creation of ozonation, which ensures that the compounds are stable and not harmful.
The use of Ultraviolet light radiations as chemical operations in wastewater treatment also employed. As argued by GOV.UK, the U.V a type of disinfectant that leaves no residues and majorly applied in the treatment of clear, un-turbid, and discoloured wastewater. Moreover, the U.V applies both high and low powered ultra violet lights wavelength of about 354nm, which ensures the killing of any pathogens, leaving no chances for microbial growth.
The calculation of the treatment dosage required achieved through an application of the equation I.
D=It where D= U.V dosage (m W.s/cm2); I= Intensity of U.V light (m W/cm2) and t= time of exposure.
Finally, the chlorination, operation as a form of chemical wastewater treatment is applied. In this process, the PH level, organic level, and wastewater temperature predict the amount of Chlorine to be added. Chlorine is added to wastewater, and it changes to both HCl and Hypochlorous acid. The formed acid- Hypochlorous reacts with toxic ammonia compounds forming less toxic compounds; chloramines as indicated by the equations (II-V) below respectively.
Cl2+H2O→H+ +Cl– + HOCl
NH3+HOCl→NH2Cl + H2O
NH2Cl+ HOCl→NHCl2+ H2O
NHCl2+HOCl→NCl3+H2O
In this process, the bacteria act on the organic matter, forming thick biomass leading to purifying the wastewater. It is also credible to appreciate that the design of the bioreactor is in such a way that it has bacteria cells, which facilitates the growth of the microorganisms.
In ensuring that the aerobic process achieved the engineering of the bioreactor, it also considers the design of the maturation ponds, basically to enable the removal of Nitrogen, Ammonia, nutrients, and pathogens since it is deep and wide constructed. The bacteria growth rate is fundamentally determined by applying the growth equation to accurately determine the amount of time that would be required to clean the wastewater through their action. The equation is shown below.
dX/dt=µ.X
Where: X=Microorganism concentration in the reactor (g/cm3)
µ=Specific growth rate (d-1)
t=time (d)
A possible combination to effectively treat the waste using the maturation operations is as shown by the diagram below.

The maturation process tends to work optimally within the normal conditions enabling more growth of the microorganisms as it requires less energy to pump Oxygen into the system to enable their growth as demonstrated by YARA.
Finally, biological wastewater treatment operation projects the amount of sludge accumulated within a certain period, indicating the amount of biomass accumulated at the bottom of the settling unit, thus predicting if some of the bacteria are still active or inactive.
If the bacteria realized to be active, the sludge returned into the bioreactor to assimilate a higher bioavailability oxygen demand (BOD) to accumulate more waste to ensure the effective utilization of the activated sludge as argued by Pullen.
Comparison and Evaluation
From the comparison of the two methods discussed above, it is evident that the chemical method appears to be more superior at the household levels than the biological method. This based on the fact that fewer resources are required to enable the process. Moreover, the design of the chemical process proves to be less complicated. This is in contrast to the biological method, which proves to be more complicated in its design and operations.
However, on the industrial scale for easier cleaning of the wastewater, there a need to consider the biological method as it proves to be more applicable and economical in that more wastewater can be treated using more cultured bacteria.
Conclusion
In conclusion, both discussed methods above (chemical and microorganisms operations) can be applied at both industrial and domestic levels to ensure water safety maintained as well addressing the water shortage-related issue meeting global demand for water. Moreover, the dimension of compliance with the water act policy, maintaining the health status of the public and effective utilization of the inadequate water is achieved.
To sum up, I believe the two approaches discussed are instrumental in the current times, which require the maximal utilization of the water resources as the level of global water sources gets demand due to the ever- rising high population.
References
- Department for Environment, Food and Rural Affairs (DEFRA). “Sewerage Treatment in the UK: UK Implementation of the EC urban Waste Water Treatment Directive.”. Web.
- GOV. “Water and treated water.”. Web.
- YARA. “Biological Wastewater treatment- Nutrients.”. Web.
- T. Pullen. “How to use recycled water for your home.”. Web.