Introduction
An inorganic polymer is a large molecule made up of a great number of inorganic monomer units that are identical to each other or at least chemically similar to each other, and are joined together in a regular way (Cowie and Arrighi 3). These units are chemically joined together with strong covalent bonds. Inorganic Polymers are formed through a process called polymerization. This is a process that involves the joining of many small molecules together to form very large molecules. These inorganic Polymers can be formed either through chain reaction polymerization or through step reaction polymerization (Daniels 11).
Inorganic polymers usually do not have carbon-carbon bonds but instead, have metal-metal bonds from a metal to oxygen like in the polysilane and polygeramnes as the main inorganic polymers for this discussion. This essay will discuss majorly the synthesis of inorganic polymers, their characteristics, what they do and how they work, their properties, their future applications, the outcomes of the procedures, which of the procedures worked and which ones did not work as well as the results of the process. The paper will enable the readers to see how a polymer is manufactured, the processes involved, the materials to be used, how the polymer is used, where it is used, and the method of applying the polymer during usage. The paper will also give a few examples of other polymers, and some of their differences in properties.
Description
Polymers can be classified as organic and inorganic polymers. Inorganic polymers are polymers that do not have carbon-carbon bonds as part of their structures. They are made of either purely metal–metal structures or metal-oxygen–metal structures. Inorganic Polymers are found and used in many ways and different areas of our lives. They also have different structures and perform different functions. A good example of a daily polymer that is known by most of us is polysilane, ploygarmane and plustannane. These are also known as polymetalloles because they are made up of many metal monomers.
Inorganic polymers such as polymetalloles and metallole–silane are used in detecting nitro atomic compounds that are usually found in explosives. So in short they are used in detecting explosives. Metalloles are polymers made up of silicon (Si) or germanium (Ge) and stanium (Sn) that contains dicyclopentadienes or dimethyl compounds. These metalloles have silicon-silicon, germanium-germanium, and stanium-stanium bonds surrounded by many conjugated organic rings which are not part of the parent chain but which majorly form side chains. They are dianions thus they have two positive charges. Their simple structures are (RC) 4Si2- and (RC) 4Ge2-, in which R represents the penile (Ph) or methyl (Me) groups in both cases.
These structures are very widely delocalized after serious X-ray experimentations. They have silicone and germanium molecules chemically joined together with strong bonds to come up with the respective macromolecules. Siloles and germoles are widely used because they have very peculiar electronic and optical characteristics and can be used in electron transportation facilities. All these things are vital components of many explosives. They are also good photoemitors and are used widely in optoelectronics. This is possible because they have an alpha–alpha electron delocalization (Hiemenz 13).
Characteristics and properties of inorganic polymers
Most inorganic polymers come with different properties and characteristics hence the reason why they are used in different ways and for different things. Some of them are hydrides of other polymers; others are a combination of two different smaller units, for example, a polymer of silicon and germanium. They tend to have different molecular formulas and molecular structures. Polymers can have different synthetic and textural properties. For instance, some polymers are hard while others are smooth; some are in powder form while others are in gel forms. Some can have high boiling points, while others come with low boiling points.
Some of these polymers have different dimensions and mechanical properties. Some have different small inorganic anions and a pair or even two pairs of delocalized electrons thus making them more effective for their usage. Others have to go through technical processes to reach the target molecule. Some need to be made in large quantities because of their massive usage while others only need to be made in small quantities. Some of these polymers are only meant for special industrial duties while others are multipurpose and can be used for more than one purpose (Hiemenz 31-33). Polysilane is one good example of generally available inorganic polymers. Its molecular structure is as shown below:
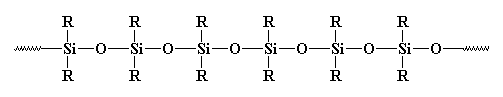
It is known that the bond connecting silicon and oxygen in the above structure is normally so strong yet can easily bend because it has some high degree of flexibility. As a result, the silicon in this structure is resistant to high temperatures with no signs of easy decaying. This makes it very valuable for its work. In the above structure R represent a Ph (phenyl) or a Me (methyl) group. So basically put the structure would look like this:
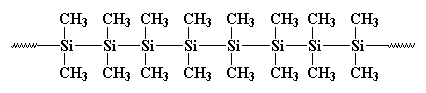
The condensed structure of polygermane is:
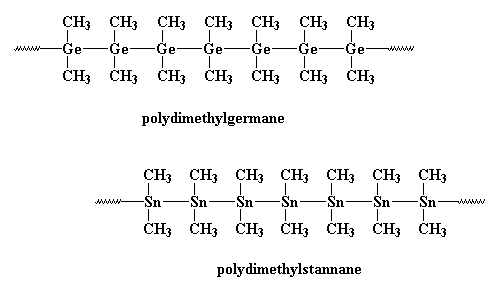
Specific Application
The process of finding or distinguishing whether or not the specimen to be examined is an explosive entails the use of photoluminescence copolymers metal-metal bond. In this case it can be Si-Si or Ge-Ge bond. It is also used by putting a thin film of the copolymer in an analyte (thus the object to be analyzed) for instance as the use of trinitrotoluene (TNT) or picric acid. This process involves finding out the slaking of the photoluminescence of the polymetalloles using toluene solution which examines the wavelengths produced from the analyte. This helps in distinguishing whether the specimen is an explosive or not.
The process of detection is based on the transfer of electrons from their excited state in the metallole copolymer up to the ground state or the lowest energy level of the analyte that is not occupied by any electrons. The electrons can be excited by using electrical methods or by optical ways. In the case of the optical methods, a source of light that has larger wavelengths may be required and this can be done using a mercury lamp or a diode that produces ultraviolet rays.
Synthesis
A traditional method of synthesizing polymetalloles and their copolymers entails the application of a method that was known as Wurtz–polycondensation. The process of making polysiloles and polygermoles together with their respective copolymers was found to be related to each other, and both used the Wurts method of polycondensation. This method of synthesis was found to be not reliable for large-scale productions since its resultant materials were very small; specifically only thirty percent.
To produce quantity and quality substances, a method that used catalytic dehydrocoupling of both metals was used. It also involved the use of Bis (cyclopentadienyl) complex forms. In this method it was found out that it is just the primary hydrosilanes that were reacted to form polysilanes in high quantities. The rest gave out dimers which were not needed in the synthesis. It was found out that the reactivity dropped drastically as substitution at the silicon atom was increased. This was because reactions that used metallocenes as their catalysts had vulnerability to steric effects. And as it is known satiric effects can always hinder reactions or even alter the reactions in one way or another (Hiemenz 40-41).
Another method of using dehydrooupling polycondensation in the presence of a catalyst was also applied. This had in it dihydro (tetraphenyl) silole and dihydro (tetrapheny) germole that had about five percent Wilkinson’s catalyst whose formula is Rh (PPh3)3Cl or Pd (PPh3)4 (Cornils 616). This method employed a technique that used polycondensation because the end result was to be a polymer and the method was to give high percentage quality yield and high volume products.
It was also a good thing to consider the cost of production as some of these can be very expensive. Silole was prepared by mixing 1,1-dichloro- 2,3,4,5-tetraphenyl silole with lithium and stirred at room temperature that is normally twenty-five to twenty-seven degrees centigrade for eight hours. One molar silane was then gently added to tetraphenyl silole dianion and mixed thoroughly by stirring for two hours.
The remaining residue that had very low boiling points (thus making them volatile) was then put under very low pressure. Finally twenty milliliters of THF was added to the resultant residue and the polymer was formed. The polymer was then taken through a gentle precipitation process by slowly adding it to seven hundred milliliters of ethanol. It was then frozen and dried and the polymers were formed as a yellow powder. As has been seen the synthesis of germole goes through the same process as that of silole. So anybody who wants to make germole only follows the same procedure but instead of silicone he or she should use germanium. The same procedure is also followed when making silole-germole polymers (Cornils 618).
The process of synthesizing polysilane is as illustrated below
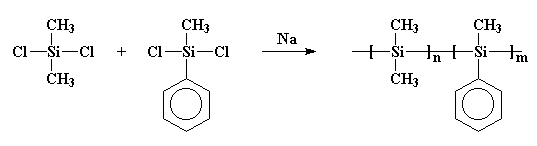
The final result is
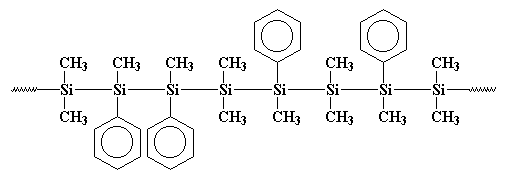
Both polygermane and polystannane follow the same procedure of synthesis.
Conclusion
In all the above cases the polymer synthesis was a total success because the procedures followed yielded the expected results. The experiments were carried out under the prescribed conditions and the necessary materials and chemicals were used. The manufactured polymers were then put to the test and were found out to be working very well. The results were also quantified and qualified and found to be at per. The best result was realized because the correct materials were used as was stipulated in the procedure. It was also discovered that the polymer can still be used shortly and does not have any unusual side effects as long as it is used expectedly. That made it cost-effective in that it not only caters to current costs but only to future costs (Cesca 57). However, everything that has a good or positive side must always have a bit of a negative side.
During the process it was discovered that the first method was not of good value as it only gave a thirty percent yield. This had to be changed and another much better process had to be adopted. This had financial implications on the plant because as is common knowledge, anything that does not yield the expected results is always a waste or a loss to an individual company or plant. It is always of importance to consider the availability of the materials to be used in the synthesis of the polymer. This saves the company a great deal of its finance as well as on the time factor (Cesca 63). It is therefore noble to come up with a plan and know how to execute the plan in a way that is going to give the desired end result without incurring huge expenses.
Works Cited
Cesca, Sebastiano. Polymers: synthesis, reactivities, properties. California: University of California Press, 1979.
Cornils, Boy. Aqueous-phase organometallic catalysis: concepts and applications. Weinheim: VCH, 2004.
Cowie, John, and Valerie Arrighi. Polymers: chemistry and physics of modern materials. New York: CRC Press, 2008.
Daniels, Carole. Polymers – structure and properties. London: Technomic Publishers, 1989.
Hiemenz, Paul. Polymer chemistry: the basic concepts. Chicago: M. Dekker, 1984.