Problem Background
The rapid expansion of hospice programs in this nation has necessitated the review of administrative programs for pain and pain management in the elderly population to ensure quality. According to the American Cancer Society, the hospice concept recognizes death as the last stage of life; it embraces life but does not attempt to expedite or postpone death (“What is hospice care,” 2019). Many studies have been conducted in recent years to analyze the impact of hospice on cost-effectiveness, benefits, and usefulness, excluding pain and pain treatment (Eshaghian-Dorcheh et al., 2020).
As a result, it is necessary to examine which types of end-of-life care treatments, administered by whom, and in what combinations perform best for certain hospice patients and their families. Other detailed studies have been conducted by reviewing patient data and conducting personal interviews with hospice personnel and relatives of dead patients. There is a need for data that addresses pain and pain management in relation to the use of non-opioid analgesics from the viewpoint of the receiver or family.
Mission, Goal, and Objectives
This study’s primary mission and goal are to evaluate the possibilities of adopting the use of non-opioid drugs in the management of pain among the geriatric population during the end-of-life palliative care. The paper will examine the current breakthroughs in the management of pain, underlying the pain medication challenges, and the potential effects of the use of opioid analgesics in palliative care in the geriatric population. Specifically, the study’s objectives are narrowed down as follows:
- Examine the health-related contribution of non-opioid drugs to palliative care in the geriatric population.
- To examine the challenges of using non-opioid drugs in pain and pain management as an intervention in hospice care.
- To assess the economic advantages of employing non-opioid medications for pain control as part of hospice care interventions in the elderly population.
Activities to Use in Implementing the Intervention
This research analysis will examine the utilization of non-opioid drugs as an effective method for pain management through a practical research approach and careful internet search analysis of the advantages and disadvantages of non-opioid medications. As such, the activities that will be discussed below will involve interviews through questionnaires on non-opioid drugs in the management of pain.
Literature Review
Opioids have traditionally been the most effective treatment for chronic pain. However, because of the associated side effects and complications, many people with chronic pain are now exploring other treatment options (Goshi et al., 2018). Healthcare practitioners in the United States and around the world have made it a priority to reduce the risks associated with chronic pain relief. According to a study by the Centers for Disease Control and Prevention (CDC), approximately one out of five Americans with cancer-related persistent pain is prescribed opioids by their physicians (Joshi et al., 2017). Opioids inhibit pain signals by blocking opioid receptors in the cerebral cortex.
Nonetheless, when one continues to counteract pain relief with incidental effect control, the body develops a tolerance to or dependence on this drug. Finding a non-opioid pain management strategy that reduces patient reliance is the most difficult challenge. There is a substantial study on the effects of long-term opioid usage on a patient’s health.
Few studies have been conducted on non-opioid alternatives, their efficacy, and their overall effects on the patient’s health. Despite this, there are writers who, in various situations and circumstances, have conducted further study on non-opioid medicines. According to Gessner et al. (2020), opioids are a time-honored standard therapy for pain. These authors attribute the widespread utilization of these medications to their low cost and immediate effectiveness.
However, the research study focuses on opioid therapy for multiple traumas. Non-steroidal anti-inflammatory medicines, acetaminophen, gabapentinoids, intravenous agents, varied degrees of local anesthetic infiltration and peripheral nerve blocks, and the more recent modality of the fascial plane block are the primary treatments employed in their study (Gessner et al., 2020). The researched modalities yielded favorable findings regarding less dependence and fewer side effects. Nevertheless, the cost and efficacy of pain treatment did not meet the expectations of the study modalities.
The following article examines non-opioid pain treatment techniques for the injured limb. Castillo (2021) analyzes the impact of gradually lowering opioid treatments while administering non-opioid medications throughout the recovery timeline of patients with severed limbs. Researchers acknowledge a research gap in this field, and their study uses realistic testing and methodologies to describe viable non-opioid alternatives to opioid therapy. In general, the research study’s findings describe the efficacy of the numerous non-opioid options for treating acute pain.
Pain and pain management are treated with non-opioid analgesics in emergency healthcare rooms. The results of non-opioid techniques in the emergency department are examined by Todd (2017). Although this form of research differs significantly from the topic of this study, there are still applicable parts that may be included in the research.
For example, Todd (2017) evaluated historical and present descriptive studies of emergency department (ED) pain management and clinical trials of developing pain treatment methods. The author evaluated the short-term effects of non-opioid pain treatment methods (Todd, 2017). Since the primary focus of the study is on the long term, the short-term consequences might be deemed unimportant. Todd (2017) examined the immediate impact of these options in emergencies and how they alleviate instantaneous pain. However, they are still essential to the study’s central argument.
Examining the efficacy of non-opioid options is crucial for a variety of reasons. Peptides are an alternative to non-opioid analgesic pain relievers. De Vega and others investigated analgesic peptides as an alternative to opioids in pain treatment (de Vega et al., 2018).
Using a descriptive qualitative study technique, De Vega et al. (2018) investigated peptides as an alternative to opioid analgesics in managing pain. Peptides target the blood’s calcium, sodium, and potassium ions. These peptides, which result from protein-protein interactions between pain-related receptors and regulatory proteins, have led to a novel approach to handling relative pain.
Methodology
Research Design
A descriptive mixed-methods approach will be applied to this project. According to Creswell (2013), the mixed method combines qualitative and quantitative approaches. Since the purpose of the study is to investigate the viability of non-opioid analgesics in pain and pain management, the researcher selected a mixed methodology.
The researcher considered that using a mixed methodology would aid in elucidating the study questions and testing the hypotheses. The quantitative part of the study will determine the frequency of use of opioid analgesics over non-opioid analgesics. The qualitative section of the study will encompass staff pain and pain management compliance with standards of hospice care.
Research Questions
The study will seek to offer answers to the underlying research concerns based on the study objectives:
- Non-opioid drugs with alternative pain management therapies provide better pain management than opioid therapy over six months?
- What challenges or risks do opioid analgesics provide over non-opioid analgesics?
- What technical improvements are required in the hospice care under the pain and pain management intervention?
Variables
Using a probability approach, the research will determine whether the independent factors, such as sex, age, and duration of non-opioid analgesic use, are statistically significant. Non-opioid analgesic exposure knowledge, human error in pain management, the safety of opioid analgesics, and the cost-effectiveness of non-opioid analgesics in pain and pain management will serve as dependent variables. The frequency will be examined to see if it is mutually exclusive, and then the basis for making predictions will be set.
Instruments
In this investigation, many research tools will be applied. The pain scale (Numeric) is the first main scale to serve as the study’s measuring pivot. The McGill pain questionnaire will acquire qualitative comparative data, such as knowledge of pain treatment options (Jumbo et al., 2020). Previous research has established the MPQ as a dependable and effective instrument for producing consistent findings (Jumbo et al., 2020). The numeric rating scale (NRS) will be retested and pre-analyzed to guarantee that the findings are valid and consistent.
Recruitment and Sampling
A group of volunteers will be asked to complete a questionnaire on the impact of opioid analgesic application in pain and palliative care pain management. The group comprising the elderly patients, guided by their families and healthcare staff, will be used in the study. As a result, a blinded, randomized research will be undertaken on a group of volunteers to assess the effect of the intervention post-test and post-survey. Priority will be given to individuals with a history of recurrent chronic or acute pain before selecting other participants; the sample size is 100 patients.
Ethical Considerations
To guarantee that the study complies with international human rights norms, it is necessary to address a variety of ethical consequences. The utilization of human volunteers to set the study’s goals is the first factor. To address this problem, participation in the research will be entirely voluntary and contingent on informed consent. The participant will have access to and comprehension of all the conditions of participation in the research project.
In addition, there is the possibility of various responses and the development of additional disorders as a result of the research medications, which presents a danger to the participants. A demographic verification mitigates this by using medical information to determine whether program participants are eligible. The advantages of the trial include free delivery of non-opioid analgesics to participants for the duration of the six-month study.
Strengths
The research on pain management using non-opioid medicines for chronic pain in hospice care will first serve as a foundation for further study in this sector. More researchers will utilize this study as a building block and a standard for future investigations. This study’s provision of hypotheses will also result in a significant decrease in the reliance on opioids for the treatment of acute or chronic pain in hospice care. Ultimately, there will be fewer adverse effects on patients, and nurses can offer patients comfort in a less invasive way.
Limitations
In this research effort, various obstacles must be overcome. The first limitation is the absence of relevant data or experience to build the investigation. Due to the many uncontrollable and unexpected aspects of a patient’s health, collecting data will likewise be challenging.
Relating the quantitative and qualitative data presented is also complex without a unifying variable that connects the two core variables. Since the study is conducted on a small scale, its breadth is also a significant obstacle. The findings cannot be confirmed for a wider group with the same health issues.
Data Collection
Intermittent data collection will be conducted before the research project and the first data collection. This data will be both qualitative and quantitative; the McGill pain questionnaire will be utilized to obtain information on pain management practices and the patients’ medical histories. After this information is acquired, quantifiable data on the patients’ progress will be collected every two months.
Data Analysis
These values will be used to compare the intersecting variables of the gathered findings using a linear regression model in SPSS version 26. Utilizing software for form modification and data analysis will boost the efficiency of data manipulation and the precision of the information. The variables of the research, including the pain dimension, sensory, emotional, evaluative, and other components, intensity, and severity of pain, will be examined using inferential statistics. Additionally, comparative statistics will be used to evaluate these factors.
Logical Model Intervention
The previous evaluation of the data recognized that the use of non-opioid analgesics in specialized hospice care for pain and pain management may be an effective, acceptable, and cost-effective method for attaining the intervention’s primary purpose. Based on the logic plan guide as indicated in Royse et al. (2016, pp. 122-127), the logic plan for this program would involve four steps.
Step 1: Gathering Helpful Information
Here, the researcher will gather pain management program records, strategic planning documents, past assessment data and reports, yearly board reports, or the program’s purpose, vision, and values.
Step 2: Assessing the Program’s Resources and Capacity
In this step, the program’s cost-effectiveness is evaluated for its efficacy. For instance, the number of staff used should relate to the funds available for the research. Assessing the capacity of the program will also assist in establishing who should comprise the core development team.
Step 3: Engaging the People Involved in the Program’s Work
Engaging several views is essential to constructing a solid logic model in this case, but numerous ways exist. It will involve facilitating sessions or workshops for the staff, interviewing the program’s management, participants, or implementers, and undertaking discussions in small groups.
Step 4: Drafting, Seeking Feedback, and Revising
A draft of the program will be created, and feedback from the program promoters will be sought to help revise all the risks and challenges that may be encountered. In addition, the study evaluation indicates that peer-based training programs for healthcare practitioners may meet the second purpose. Consequently, the suggested intervention will consist of two operational components.
First, it will establish the non-opioid analgesics-recommended intervention at one of the specialist hospice care facilities, with non-compliance monitored by a healthcare facilitator. The best design and schedule for the proposed intervention and training program will consist of a biweekly educational session with post-intervention evaluations every three months for six months. For the quantitative nature of the research, the numeric pain rating scale will be used to assess the patients’ pain levels over six months before and after the administration of non-opioid analgesics.
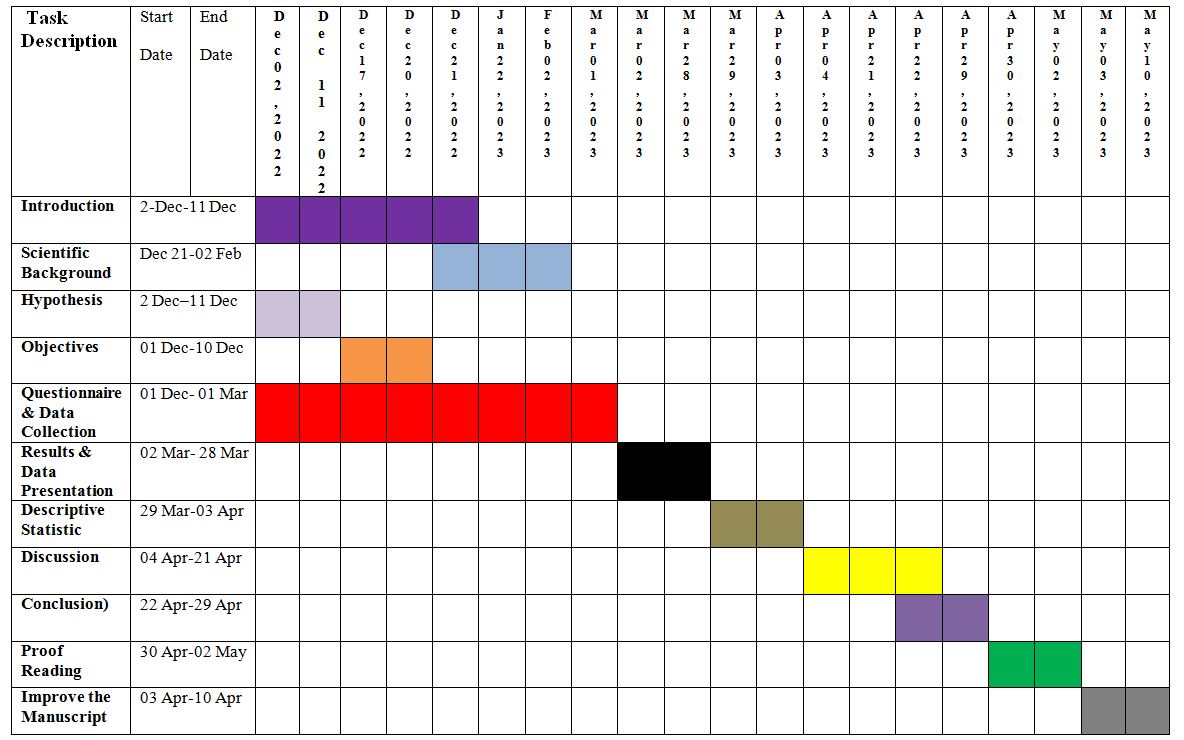
Table 1: The Gantt chart
Staffing Plan
Staffing Plan
The goal of the staffing plan is to make sure that enough people with the skills and experience needed for the project are assigned to it so that it can be finished successfully.
Role Requirements
The responsibilities necessary to complete the project’s activities are detailed below. The following information is provided for each key project activity: the project position, the role’s responsibilities, the number of personnel needed to perform each function, and the anticipated start and end dates for the project’s staff resource requirements. A total of 29 personnel will be used in this evaluation plan.
The project owner will provide the scientific background, objective, and hypothesis writing. Since the research uses a questionnaire, the views on the program will be derived from 20 patients with direct impact of pain. Moreover, because of the pain, answering the questionnaire might require further assistance from staff; therefore, eight personnel will be provided.
Table 2: Staffing requirement plan
Budget for the Evaluation Plan
Table 3: Budget plan for the project
References
Castillo, R. C., & McGinnis, A. (2021). Non-opioid strategies for pain management of the mangled limb. In The Mangled Extremity (pp. 213-223). Springer. Web.
de Vega, M. J. P., Ferrer-Montiel, A., & González-Muñiz, R. (2018). Recent progress in non opioid analgesic peptides. Archives of Biochemistry and Biophysics, 660(1), 36-52. Web.
Eshaghian-dorcheh, A., Zandi, M., Rasouli, M., Tahmasebi, M., & Esmaielzadeh, F. (2020). Evaluating the cost-effectiveness of home-based palliative care for children with special health care needs: a review study. International Journal of Pediatrics, 8(11), 12381-12395. Web.
Gessner, D. M., Horn, J. L., & Lowenberg, D. W. (2020). Pain management in the orthopaedic trauma patient: Non-opioid solutions. Injury, 51(2), 28-36. Web.
Joshi, G. P., Kehlet, H., Beloeil, H., Bonnet, F., Fischer, B., Hill, A., Lavandhomme, P.M., Lirk, P., Pogatzki-Zhan, E.M., Raeder, J., Van de Velde, M., & Rawal, N. (2017). Guidelines for perioperative pain management: need for re-evaluation. British Journal of Anaesthesia, 119(4), 720-722. Web.
Jumbo, S. U., MacDermid, J. C., Kalu, M. E., Packham, T. L., Athwal, G. S., & Faber, K. J. (2020). Measurement properties of the Brief Pain Inventory-Short Form (BPI-SF) and the Revised Short McGill Pain Questionnaire-Version-2 (SF-MPQ-2) in pain-related musculoskeletal conditions: a systematic review protocol. Archives of Bone and Joint Surgery, 8(2), 131 –141. Web.
Royse, D., Thyer, B.A., Padgett, D.K. (2016). Program evaluation: An introduction (6th Ed.). Wadsworth/Thompson
Todd, K. H. (2017). A review of current and emerging approaches to pain management in the emergency department. Pain and Therapy, 6(2), 193-202. Web.
What is hospice care? (2019). American Cancer Society. Web.